The COVID-19 pandemic had a significant impact on the market studied. Due to the emergence of COVID-19, the demand for air filters increased globally. There was a rising awareness regarding air filtration, and the use of masks containing filters increased among the population. This increased awareness created a huge demand for the development of various types of filters and brought new research and innovation to the market. For instance, as per an article published in Springer Nature in October 2021, HEPA air cleaners or filters are a cheap and easy way to reduce the risk of airborne pathogens. And it was also mentioned in the article that when a cost-effective portable filter containing a HEPA filter was used in hospital settings in the United Kingdom, it efficiently screened SARS-CoV-2 and other disease-causing microorganisms from hospital air. Thus, such instances created a demand for the use of filters during the pandemic, which ultimately generated demand for filter integrity tests done to check the integrity of the filters. Therefore, owing to the factors mentioned above the pandemic had a considerable impact on the market studied and is expected to project a similar trend in the post-pandemic owing to increased awareness regarding filter integrity, as per the analysis.
Factors such as increasing demand for biopharmaceuticals and higher spending on research and development (R&D) activities majorly contribute to the studied market growth.
The number of manufacturers is increasing in the biopharmaceutical sector due to the increasing demand for biopharmaceuticals. As per an article published in FPHAR in August 2022, biopharmaceuticals are one of the potential achievements of the twenty-first century and are superior to chemical products in terms of disease management and treatment outcomes. Additionally, as per the source mentioned above, biopharmaceuticals contain few to no adverse effects, and they exert more activity and specificity than conventional drugs. In addition, the development of biopharmaceutical capabilities is typically considered a country’s priority and an indicator of scientific advancement. Thus, investments in the development of biopharmaceuticals are heavily influenced by the high demand for their availability, ultimately requiring aseptic conditions for manufacturing. Thus, the demand for filters in such facilities is likely to rise owing to the aseptic conditions required, in turn boosting the installation and adoption of several filters. Such an increased demand for filters will boost the adoption of filter integrity tests across biopharmaceutical manufacturing companies, leading to market growth.
In addition, a few other sources reviewed stated that integrity testing is a non-destructive test that is directly tied to a filter's capacity to retain microorganisms and gives assurance of filter performance in the customer's process. Integrity testing enables manufacturers to satisfy auditor requirements while avoiding costly problems including product contamination, product losses, reprocessing, and production delays. Thus, the use of filter integrity tests is increasing among biopharmaceutical manufacturers. Hence, with the increasing demand for biopharmaceuticals, the demand for filter integrity testing is increasing, driving the studied market's growth.
Furthermore, market players are adopting various strategies, such as product launches, collaborations, acquisitions, mergers, and expansions, to increase market share and expand their presence. For instance, in December 2021, Pall Corporation, in association with Express Pharma, introduced its new Palltronic Flowstar V filter integrity test instrument in India. This instrument is a filter integrity test device that supports critical filtration steps and batch release while addressing good manufacturing practice (GMP) manufacturing demands. Such developments in the market are anticipated to fuel market growth over the forecast period.
Therefore, owing to increasing biopharmaceutical manufacturing and the continuous involvement of market players, the studied market is anticipated to witness growth over the forecast period. However, the high cost of tests is expected to hamper the market growth.
Filter Integrity Test Market Trends
Automated Segment is Expected to Witness Growth Over the Forecast Period
Filter integrity testing is done to ensure that the final product is free of contamination. This involves examining the disc filters, capsules/cartridges, and filter membranes for integrity. Automated integrity test systems or machines are available in either a standalone or networkable configuration and offer reliable, repeatable integrity test data as opposed to manual filter integrity tests. Thus, owing to such an advantage, the demand for automated systems is expected to increase, further fueling the segment's growth.Few of the commercially available automated systems provide better reliability. Merck Millipore's Integritest 4 series automated filter integrity test instrument, for example, features an intuitive touch screen and user interface that streamlines the test process and accelerated testing capability that gives users more time for processing. Additionally, on-site calibration support and the diagnostic capability of the machine minimize downtime, saving time and money.
In addition, several other automated systems available on the market perform various filter integrity tests. For example, the Sartocheck 5 Plus Filter Tester is an automatic filter integrity tester that can test everything from small syringe filters to large multi-round housings and tangential flow cassettes. This is prepared for automated cleaning of all pneumatics as well. Thus, the availability of such advanced automated machines is driving the segment's growth.
Therefore, due to the market's availability of advanced automated systems, the segment is expected to witness growth over the forecast period.
North America is Anticipated to Witness Growth Over the Forecast Period
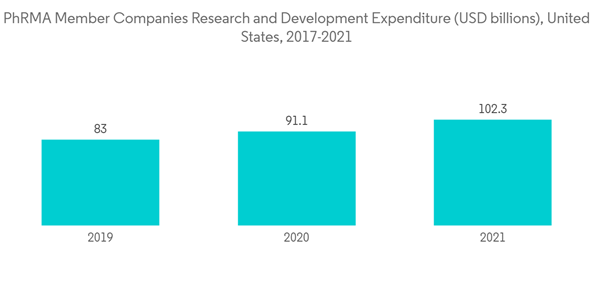
North America is holding the largest share of the market, and it is expected to maintain its stronghold over the analysis period. Factors such as high R&D expenditure and increasing innovation and development of biopharmaceuticals across the countries of the region are majorly attributed to the growth of the market in North America.Before batch processing, filter integrity is monitored during integrity testing of sterilizing filters, preventing the use of a non-integral filter. After a batch of filters has been sterilized, their integrity can be tested to see if it has been compromised. Thus, with the increasing demand for pharmaceuticals and biopharmaceuticals, R&D expenditure is also expected to augment in the United States and other countries across the region, ultimately driving the studied market growth. As per the data updated by PhRMA member companies' annual survey data released in July 2022, PhRMA member companies invested USD 102.3 billion in R&D in 2021, the highest level of investment ever made in the United States.
Furthermore, the spending on R&D by various pharmaceutical companies along with government organizations has been increasing recently across the countries in Canada, which is anticipated to drive market growth in the region. For instance, the government and private sectors are heavily investing in pharmaceutical drugs. The November 2021 update by CIHI shows that healthcare expenditure was USD 230,005 million by the public sector and USD 78,038.3 million by the private sector in 2021, which increased from USD 226,246.5 million and USD 75,208.3 million by the public and private sectors, respectively, in 2020. Thus, this increasing healthcare expenditure in the country is predicted to impact the demand for biopharmaceuticals, in turn boosting productivity across the facilities that make use of filters for GMP.
In addition, as per the source above, in-house spending on R&D is estimated to grow by 3.5%, reaching USD 23.4 billion in 2021. While the companies expect modest growth in their in-house R&D spending in 2022, total R&D spending is expected to increase by 1.7% to USD 23.8 billion (estimated). Thus, these increasing investments by governments across Canada are creating demand for the development of advanced drugs for various purposes. This is fueling the demand for filter integrity tests to check drug safety and efficacy. Hence, it is anticipated to drive the studied market's growth.
Therefore, owing to an increase in investments, North America is expected to hold a major market share over the forecast period.
Filter Integrity Test Market Competitor Analysis
The filter integrity test market is competitive and consists of several major players. Some companies currently dominating the market are Merck KGaA, Sartorius AG, Meissner Filtration Products Inc., ThermoFisher Scientific Inc., Donaldson Company, Parker Hannifin Corp., Eaton Corporation PLC, Pall Corporation, 3M, and Filter Integrity Ltd., among others.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 3M
- Donaldson Company
- Eaton Corporation PLC
- Filter Integrity Ltd
- Meissner Filtration Products Inc.
- Merck KGaA
- Pall Corporation
- Parker Hannifin Corp.
- Sartorius AG
- ThermoFisher Scientific