The healthcare system witnessed enormous challenges as a result of the COVID-19 pandemic. During the first phase of COVID-19, the expansion of the patient-controlled analgesia pump market was significantly constrained for several uncertain reasons. However, there is an increasing occurrence of chronic pain in patients who recovered from COVID-19 infection. For instance, as per the Current Pain and Headache Reports in March 2022, the vaccination for COVID-19 in the United States may be reducing the number of new SARS-CoV-2 cases, but the population of those suffering from the virus's long-term symptoms is growing. One of these long-term side effects includes chronic pain syndromes. Hence, to manage the chronic pain of COVID-19-recovered patients, there is a need for patient-controlled analgesia pumps, which help the patient control their pain. This is expected to positively impact the market over the forecast period.
The major factors attributing to the market's growth are the increasing prevalence of chronic pain diseases such as arthritis, cancer, and fibromyalgia, along with the technological advancements with enhanced safety features in the PCA pumps. Fibromyalgia is a condition that causes pain all over the body, also called widespread pain. The increase in the prevalence of fibromyalgia helps the growth of the patient-controlled analgesia pump market. For instance, according to the updated data from CDC in May 2022, about 4 million US citizens, or 2% of all adults, suffer from fibromyalgia. Although there is no known cause for fibromyalgia, it is treatable and manageable with various pain management methods, one of which is a patient-controlled analgesia pump. Hence a significant burden of chronic pain diseases like fibromyalgia increases the usage of patient-controlled analgesia pumps and drives the market's growth over the study period.
The recent product launches and approvals by the regulatory authorities focusing on patient-controlled analgesia pumps are expected to aid in the market's growth, as these approvals will increase product availability and competition in the market. For instance, in April 2021, Becton, Dickinson, and Company submitted a 510(k) premarket notification to the US FDA for the BD Alaris System. The system allows clinicians to deliver pain medications and fluids through a single integrated platform that includes large-volume pumps and syringe pumps for adult, pediatric and neonatal patients. Thus, the large population affected by chronic pain-causing diseases and the increasing product approvals are expected to add to the growth of the studied market over the forecast period. However, the lack of awareness resulting in patient medication errors and the stringent regulatory framework and associated product recalls are likely to slow down the market growth over the forecast period.
Patient-controlled Analgesia Pumps Market Trends
Oncology is Expected to Hold the Major Market Share in the Market Over the Forecast Period
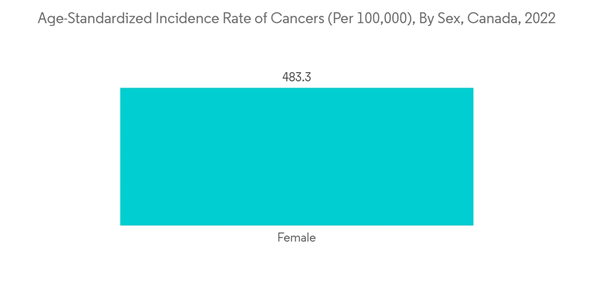
The oncology segment is expected to account for a significant market share during the forecast period due to the widespread prevalence of cancers worldwide. Cancer pain is often described as a throbbing, pressing, burning, or tingling sensation that can be managed through PCA pumps, and hence, the demand for PCA pumps is expected to increase with the rising burden of cancer. For Instance, according to the Canadian Cancer Statistics 2021, in Canada, 229,200 new cancer cases were detected in 2021. Similarly, as per the report published by the CMAJ Group in 2022, an estimated 233,900 people in Canada will be diagnosed with cancer in 2022. Thus, the increase in new cases of cancer in the region will increase the demand for pain management, which can be achieved using patient-controlled analgesia pumps. Therefore, growth in the market is expected. Highly potent opioid drugs, such as hydro morphine, morphine, and fentanyl through patient-controlled analgesia (PCA) infusion, are prescribed by physicians to treat unbearable pain. The rising adoption of PCA pumps in such conditions to improve the quality of life is expected to promote the segment's growth.Similarly, the increase in patient satisfaction with cancer pain management with the use of patient-controlled analgesics plays a significant role in market growth. For instance, as per an article published by the JNCCN Journal in August 2021, the programmable patient-controlled analgesia (PCA) pump allows patients to control pain on demand and also promptly responds to the patient's changing needs, and it reduces analgesic delay. The PCA significantly reduced successful titration and average pain score for persistent severe cancer pain. Thus, the preference for PCA pumps for cancer pain management is expected to boost the growth of the segment.
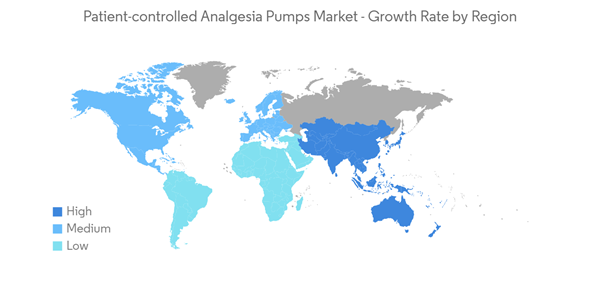
North America is Expected to Hold a Significant Share in the Market Over the Forecast Period
North America is expected to dominate the overall PCA pumps market owing to the rise in the number of cases of cancer, accidental injuries, and other ailments related to pain and the rise in research funding on chronic pain.The high prevalence of cancer cases in the North American region is expected to add to the market's growth. For instance, as per the ACS 2021 & 2022 report, in the United States, 1,898,160 new cancer cases were estimated in 2021, which is going to rise in 2022 to 1,918,030 new cancer cases. Excluding non-melanoma skin cancer, 42% of cancer cases in the United States that are newly diagnosed, or around 805,600 cases in 2022, may be preventable. Thus, the rising prevalence of cancer cases in the region is likely to boost the market's growth in the region over the forecast period.
Furthermore, the rise in research funding on chronic pain is also expected to propel the market's growth in the region. For instance, as per NIH 2022, the research funding for chronic pain in the United States for 2021, 2022, and 2023 is estimated at USD 725 million, USD 750 million, and USD 862 million, respectively. In addition, the partnerships and acquisitions among the key market players in the region are also adding to the market's growth. For instance, in January 2022, California-based ICU Medical acquired the Smiths Medical Division, which includes syringe and ambulatory infusion devices, vascular access, and vital care products from Smiths Group Plc. Such activities are likely to expand the portfolio of companies, which are expected to witness significant growth over the forecast period in the North American region.
Patient-controlled Analgesia Pumps Market Competitor Analysis
The PCA pumps market is moderately competitive and consists of several major players. In terms of market share, a few of the major players are currently dominating the market. Some prominent players are developing safe and trouble-free systems with novel technologies, while others are distributing them. Some of the companies that are currently dominating the market are ICU Medical Inc. (Smiths Medical), BD (Becton, Dickinson and Company), Fresenius SE & Co. KGaA, B. Braun SE, and Baxter.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ICU Medical Inc. (Smiths Medical)
- Baxter
- BD (Becton, Dickinson and Company)
- Fresenius SE & Co. KGaA
- Micrel Medical Devices
- Avante Health Solutions
- B. Braun SE
- Ace Medical
- Terumo Corporation
- Abbott
- Pfizer Inc. (Hospira)