The majority of pharmaceutical and biotech companies are concentrating their research & development departments on finding new compounds or leads for the treatment of this disease, which is in the last stages of clinical trials, as a result of the COVID-19 pandemic. By providing funding and incentives to the pharmaceutical corporations, the local government further supports those clinical trials. The Danish Ministry of Health awarded 18.8 million Danish Kroner in November 2020 to test a novel coronavirus vaccine created by scientists at the nation's State Serum Institute (SSI). In order to guarantee that the vaccination is accessible to around 2 million individuals in Europe, Denmark also agreed to a fourth contract with BioNTech/Pfizer in November 2020. BioNTech/Pfizer is now thought to be a prospective vaccine manufacturer. Thus, in the long term, the market is expected to grow.
One of the foundational areas of the Danish economy is the pharmaceutical sector. Compared to other European nations, the nation boasts a sizable pharmaceutical and biotechnology sector, as well as a number of indigenous enterprises that conduct pharmaceutical product development and production. Pharmaceutical businesses have been making innovations including strategic mergers, collaborations, and investments in order to take advantage of the prospects that are currently present and in the future. In order to supplement its ongoing clinical activities in immuno-dermatology, UNION therapeutics A/S inked an agreement in July 2020 to purchase LEO Pharma A/global S's rights to the LEO PDE4 inhibitor chemical series. The substance was given the new name 'UNI500' and is a class of PDE4 inhibitors. In June 2020, Fujifilm Corporation announced a new significant capital investment in the Danish location of FUJIFILM Diosynth Biotechnologies, the country's life science industry, totaling roughly 100 billion yen, or USD 928 million. The company hopes to increase its present contract research and production organisation for biologics and advanced therapeutic capabilities with this new capital investment from FUJIFILM Diosynth Biotechnologies. AbbVie Inc. and Genmab A/S, a Danish biotechnology company, entered into a cooperation agreement in June 2020 under which AbbVie would make an upfront payment of USD 4 billion to develop and market a number of Genmab's early-stage cancer medicines. Genmab will be able to expand its research and development pipeline with the aid of this milestone payment. Thus, the abovementioned factors are expected to increase the market growth.
However, the high tax rates on pharmaceutical products and price-cap agreements are expected to hinder the market growth.
Denmark Pharmaceutical Market Trends
Prescription Drugs (Rx) accounted for the Significant Share of the Total Pharmaceutical Sales in Denmark
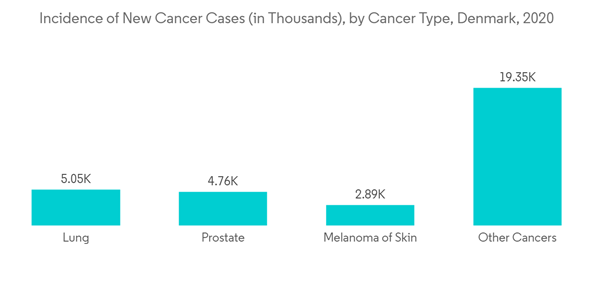
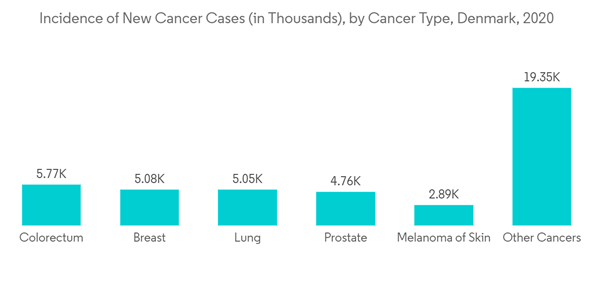
In Denmark, the prescriptions are not covered under public health insurance. However, the national scheme does reimburse the partial costs of some medications. Furthermore, in Denmark, the direct-to-consumer advertising of prescription drugs is permitted under strict legislation. This is having a positive impact on the market as most pharmacists and end-users will be well aware of new or present medications available for the treatment of diseases. Prescription drug sales have increased as compared to previous years owing to the rising chronic disease.According to Globocan 2020, Denmark has an estimated 42,891 incidents of cancer. Colorectal cancer is the most common type of cancer, followed by breast, lung, prostate, and skin melanoma. Major competitors in the prescription drug market are making substantial advancements for the unmet requirement that exists in the market space as a result of the rising prevalence of chronic diseases in this area. For instance, a major player in this market, Novo Nordisk, is conducting a clinical trial comparing once-weekly semaglutide 2.0 mg to once-weekly semaglutide 1.0 mg. In November 2020, the company announced that the SUSTAIN FORTE trial, a 40-week, phase 3b, efficacy and safety trial with once-weekly semaglutide 2.0, had demonstrated a statistically significant and superior reduction in HbA1c at week 40. The business wants to market the medicine and get additional regulatory approval. Similarly, the world's largest diabetic manufacturer, Novo Nordisk, purchased Corvidia Therapeutics from AstraZeneca in June 2020 for an initial USD 725 million as it aims to diversify into cardiovascular disease medicines in the Danish market. Thus, the abovementioned factors are expected to increase the market growth.
Denmark Pharmaceutical Market Competitor Analysis
The pharmaceutical industry is competitive and consists of several major players in Denmark having a major focus on research and development. Some of the major pharmaceutical companies in the country are Novo Nordisk A/S, H. Lundbeck A/S, Leo Pharma A/S, Orifarm Group A/S, ALK-Abelló A/S, Xellia ApS, Takeda Pharma A/S, Sandoz A/S, Ferring Pharmaceuticals A/S, and FUJIFILM Diosynth BiotechnologiesAdditional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Novo Nordisk A/S
- H. Lundbeck A/S
- Leo Pharma A/S
- Orifarm Group A/S
- ALK-Abell A/S
- Xellia ApS
- Takeda Pharma A/S
- Sandoz A/S
- Ferring Pharmaceuticals A/S
- FUJIFILM Diosynth Biotechnologies