Ventricular assist devices (VADs) are medical devices designed to support the functioning of a weakened or failing heart. They are primarily used as a temporary measure while a patient awaits a heart transplant or as a long-term solution for patients who are not eligible for transplantation. VADs are implanted surgically and help pump blood from the heart's ventricles to the rest of the body. These devices consist of a pump, which is typically placed in the patient's abdomen or chest, and a driveline that connects the pump to an external power source. The pump is responsible for taking over the pumping action of the heart, ensuring that blood is adequately circulated throughout the body. They can help relieve symptoms, including fatigue, shortness of breath, and fluid retention. Moreover, VADs can serve as a bridge to transplantation, keeping patients stable and improving their chances of receiving a donor heart. As a result, VADs have revolutionized the treatment of heart failure, providing patients with improved quality of life, higher energy levels, and increased survival rates.
A rise in the prevalence of cardiovascular diseases (CVDs) represents one of the key factors driving market growth. Besides this, the growing awareness among healthcare professionals and patients about the benefits and availability of VAD therapy is contributing to the market expansion. A surge in efforts to educate healthcare providers, patients, and caregivers about the effectiveness of VADs in managing heart failure has led to increased acceptance and adoption of these devices. In addition, supportive regulatory frameworks and approvals have facilitated the commercialization and adoption of VADs. Regulatory agencies worldwide have been actively involved in streamlining the approval process, ensuring safety, and promoting innovation in VAD technology. Such favorable regulatory environments encourage manufacturers to invest in research and development (R&D) and introduce advanced VADs. Additionally, advancements in surgical techniques for VAD implantation have made the procedure safer and more accessible. The introduction of minimally invasive (MI) surgical approaches, such as thoracotomy or subcostal incisions, has reduced surgical trauma, shortened recovery times, and expanded the eligibility of patients for VAD therapy, which in turn has accelerated the adoption of VADs. Furthermore, the increasing healthcare expenditure, particularly in emerging economies, is contributing to the growth of the VADs market. Other factors, including significant economic growth, improving healthcare infrastructure, favorable government initiatives to enhance healthcare access, and the rising willingness of patients to invest in novel treatment options for heart failure, are also anticipated to stimulate market growth.
Ventricular Assist Devices Market Trends/Drivers
Increase in the prevalence of heart failure
The growing prevalence of heart failure across the globe is a significant factor driving the market for ventricular assist devices. The rise in heart failure cases can be attributed to the aging population, sedentary lifestyles of individuals, obesity, and an increase in chronic diseases such as diabetes and hypertension. The risk of heart failure rises with age, and older adults are more likely to require mechanical circulatory support. The aging population, especially in developed countries, creates a substantial patient pool for VADs, fueling market growth. As the number of heart failure patients continues to grow, the demand for VADs as a viable treatment option also increases. VADs offer a solution for individuals with advanced heart failure who may not respond adequately to conventional therapies, providing mechanical support and improving their quality of life.Limited availability of donor hearts
The scarcity of suitable donor hearts for transplantation is another critical factor strengthening the market growth. Heart transplantation remains the gold standard treatment for end-stage heart failure. However, the number of available donor hearts falls significantly short of the demand. Since VADs serve as a bridge to transplantation, keeping patients stable and improving their chances of receiving a donor heart, they are increasingly being used as a long-term solution for patients who are not eligible for transplantation. As a result, the limited availability of suitable heart donors has generated the need for alternatives to heart transplantation, such as VADs, which is further expected to fuel the growth of the market during the forecast period.Rise in technological advancements
Rapid advancements in VAD technology have significantly contributed to the growth of the market. Continuous research and development (R&D) efforts by key players have led to smaller device sizes, improved durability, enhanced control systems, and better patient outcomes. These technological advancements have expanded the use of VADs to a broader patient population, including those with smaller body sizes or children with heart failure. Moreover, advancements in device design and implantation techniques have reduced the risk of complications, increased device reliability, and improved patient comfort. Such innovations and improvements in VAD technology have led to the development of innovative devices that are more efficient, safer, and appealing to both healthcare professionals and patients, thereby supporting market growth.Ventricular Assist Devices Industry Segmentation
This report provides an analysis of the key trends in each segment of the global ventricular assist devices market report, along with forecasts at the global, regional and country levels from 2025-2033. The report has categorized the market based on product, flow type, product type, application, and end user.Breakup by Product
- Left Ventricular Assist Device (LVAD)
- Right Ventricular Assist Device (RVAD)
- Biventricular Assist Device (BiVAD)
- Others
The report has provided a detailed breakup and analysis of the market based on the product. This includes left ventricular assist device (LVAD), right ventricular assist device (RVAD), biventricular assist device (BiVAD), and others. According to the report, left ventricular assist device (LVAD) represented the largest segment.
Breakup by Flow Type
- Pulsatile Flow
- Non-Pulsatile or Continuous Flow
The report has provided a detailed breakup and analysis of the market based on the flow type. This includes pulsatile flow and non-pulsatile or continuous flow. According to the report, non-pulsatile or continuous flow represented the largest segment.
Breakup by Product Type
- Implantable Ventricular Assist Devices
- Non-implantable Ventricular Assist Devices
The report has provided a detailed breakup and analysis of the market based on the product type. This includes implantable ventricular assist devices and non-implantable ventricular assist devices. According to the report, implantable ventricular assist devices represented the largest segment.
Breakup by Application
- Bridge-to-Transplant (BTT) Therapy
- Destination Therapy
- Bridge to Recovery and Bridge to Candidacy
The report has provided a detailed breakup and analysis of the market based on the application. This includes bridge-to-transplant (BTT) therapy, destination therapy, and bridge to recovery and bridge to candidacy. According to the report, bridge-to-transplant (BTT) therapy represented the largest segment.
Breakup by End User
- Ambulatory Surgery Centers
- Hospital
- Others
The report has provided a detailed breakup and analysis of the market based on the end user. This includes ambulatory surgery centers, hospital, and others. According to the report, hospital represented the largest segment.
Breakup by Region
- North America
- United States
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
The report has also provided a comprehensive analysis of all the major regional markets, which include North America (the United States and Canada); Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa. According to the report, North America was the largest market for ventricular assist devices.
The report has provided a comprehensive analysis of the competitive landscape in the market. Detailed profiles of all major companies have also been provided. Some of the key players in the market include:
- Abbott Laboratories
- Abiomed Inc.
- Berlin Heart GmbH (Syscore GmbH)
- Bivacor Inc.
- Calon Cardio
- Cardiacassist Inc. (LivaNova PLC)
- CHF Solutions Inc.
- Jarvik Heart Inc.
- MAQUET GmbH (Getinge)
- Medtronic Inc.
- Syncardia Systems LLC (Versa Capital Management LLC)
- TandemLife (LivaNova PLC)
- Terumo Corporation
Key Questions Answered in This Report
1. How big is the global ventricular assist devices market?2. What is the expected growth rate of the global ventricular assist devices market during 2025-2033?
3. What are the key factors driving the global ventricular assist devices market?
4. What has been the impact of COVID-19 on the global ventricular assist devices market?
5. What is the breakup of the global ventricular assist devices market based on the product?
6. What is the breakup of the global ventricular assist devices market based on the flow type?
7. What is the breakup of the global ventricular assist devices market based on the product type?
8. What is the breakup of the global ventricular assist devices market based on the application?
9. What is the breakup of the global ventricular assist devices market based on the end-user?
10. What are the key regions in the global ventricular assist devices market?
11. Who are the key players/companies in the global ventricular assist devices market?
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Abiomed Inc.
- Berlin Heart GmbH (Syscore GmbH)
- Bivacor Inc.
- Calon Cardio
- Cardiacassist Inc. (LivaNova PLC)
- CHF Solutions Inc.
- Jarvik Heart Inc.
- MAQUET GmbH (Getinge)
- Medtronic Inc.
- Syncardia Systems LLC (Versa Capital Management LLC)
- TandemLife (LivaNova PLC)
- Terumo Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 149 |
| Published | February 2025 |
| Forecast Period | 2024 - 2033 |
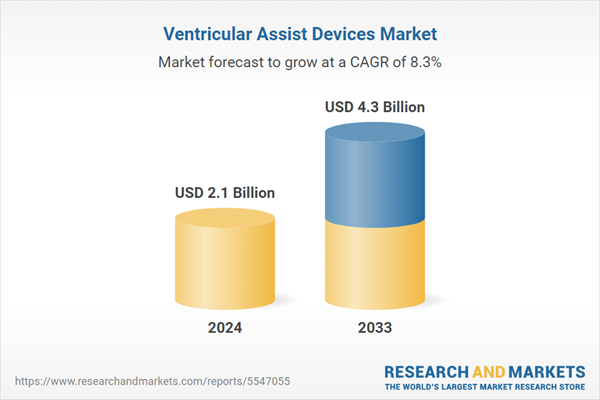
| Estimated Market Value ( USD | $ 2.1 Billion |
| Forecasted Market Value ( USD | $ 4.3 Billion |
| Compound Annual Growth Rate | 8.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |