Dermatology is a field of medicine that deals with diseases of the skin, hair, and nails, with psoriasis, eczema, and acne vulgaris being the most common. Dermatology medications are used to treat and manage diseases of the skin, nails, hair, and genital membranes. Due to reasons such as nutritional inadequacy, bad eating habits, increased stress, pollution, and a lack of immunity in the worldwide population, the pool of people affected by dermatological problems has rapidly expanded in recent years.
The demand for appropriate treatment methods and dermatological medications for the treatment of dermatological disorders has risen as a result. The global dermatological drug market is segmented by indication, route of administration, end-user, and geography.
Market Drivers:
The increasing prevalence of dermatological conditions and the rise in research and development in healthcare are anticipated to surge the market.
One of the most important factors driving the dermatological medication industry is the rising prevalence of skin diseases. Trauma, environmental and hereditary factors, and age are only a few of the major variables that contribute to the development of skin diseases in millions of people around the world. Since 2013, World Skin Health Day has been observed around the world to raise awareness of and treat a variety of skin conditions. According to the World Health Organization (WHO), skin diseases are one of the most common types of health disorders, affecting nearly 900 million people worldwide at any given time.The healthy functioning of the human skin begins to deteriorate with age, leading to an incidence of various skin diseases, propelling the dermatology drug market forward. Furthermore, as people get older, they begin to experience a variety of other issues, including slower wound healing, increased sensitivity to ultraviolet (UV) radiation, increased susceptibility to infections, and a loss of subcutaneous fat. According to Australian research, acne vulgaris affects more than 4% of the population in the long run. As a result, the market is expected to develop at a faster rate during the projected period due to an increase in the frequency of skin diseases and the demand for effective medications.
Acne is the most common skin disorder, according to the American Academy of Dermatology, impacting 40 to 50 million people in the United States each year. It is more common in younger people, with 85% of people aged 12 to 24 suffering from some kind of acne. According to the National Rosacea Society, the rosacea patient population is rising, with 16 million people in the United States alone suffering from the condition. The worldwide dermatological pharmaceutical market is likely to be impacted further by the regular release of novel and customer-centric products. For example, Sol-Gel Technologies announced FDA clearance of TWYNEO in July 2021. It's a topical cream for the treatment of acne vulgaris in adults and children aged 9 and above.
Market Restraint:
Risks of negative effects from incorrect product use can act as a limitation on the market for dermatological drugs.
Emerging countries account for a large share of the global population with dermatological infections owing to poor diet, pollution, and unhealthy habits such as smoking and drinking. Lack of information on skin problems, as well as a higher illiteracy rate, have resulted in decreased adoption of dermatological treatments in low-middle-income nations. The negative effects connected with the products are another issue impeding the market’s growth. For example, the US Food and Drug Administration (FDA) has issued an advisory regarding the use of certain over-the-counter drugs that might induce life-threatening allergic reactions. This is anticipated to reduce product adoption, resulting in a drop in the market value of dermatology medications.North America is expected to hold a significant share of the global market.
Due to the simple availability of dermatological drugs, North America is estimated to account for a large proportion of the dermatological drug market over the forecast period. Furthermore, an increase in the occurrence of skin ailments such as dermatitis, acne, and psoriasis is a prominent factor driving this market's rise. On the other hand, due to increased awareness about the usage of dermatological medications,As technology advances, there is a rise in public knowledge about the usage of dermatological medications. Furthermore, it helps with the expansion of the dermatological medication market by increasing people's aesthetic sensibility.
Key Developments:
- December 2023-Arcutis Biotherapeutics received FDA approval for its roflumilast cream (ZORYVE) 0.3% topical foam, a topical phosphodiesterase-4 inhibitor, for plaque psoriasis in patients aged 12 and above and seborrheic dermatitis in individuals aged 9 and older. ZORYVE foam is a safe, effective, and well-tolerated once-daily steroid-free foam for use on all affected areas, including hair-bearing areas. It provides rapid disease clearance and a significant reduction in itch, a common symptom of seborrheic dermatitis.
- January 2023-Bristol Myers Squibb received a positive CHMP opinion for Sotyktu, an oral medication for adults with moderate-to-severe plaque psoriasis. Sotyktu, a selective TYK2 inhibitor, showed significant improvements in skin clearance, symptom burden, and quality of life compared to placebo and Otezla. The recommendation reviewed by the European Commission. The drug was well tolerated, with a low rate of discontinuation due to adverse events. The FDA approved Sotyktu in September 2022, while Japan's Ministry of Health, Labour and Welfare approved it for adults with plaque psoriasis, generalized pustular psoriasis, and erythrodermic psoriasis who have had inadequate response to conventional therapies.
- January 2022-The U.S. Food and Drug Administration (FDA) approved RINVOQ® (upadacitinib) for treating moderate to severe atopic dermatitis in adults and children aged 12 years and older. The approval is based on efficacy and safety data from a Phase 3 program involving over 2,500 patients. The study showed significant improvement in itch as early as week one and skin clearance at 16 weeks compared to the placebo. In the U.S., RINVOQ 15 mg and 30 mg are approved for use in adults and pediatric patients with refractory, moderate to severe atopic dermatitis.
Key Market Segments:
By Indication
- Psoriasis
- Acne
- Dermatitis
- Others
By Route Of Administration
- Topical
- Oral
- Parenteral
By End-User
- Hospitals And Clinics
- Cosmetic Centers
- Others
By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- Germany
- France
- United Kingdom
- Spain
- Others
- Middle East and Africa
- Saudi Arabia
- South Africa
- Israel
- Others
- Asia Pacific
- China
- Japan
- Thailand
- Indonesia
- South Korea
- India
- Taiwan
- Others
Table of Contents
Companies Mentioned
- AbbVie Inc
- Amgen Inc
- GlaxoSmithKline Plc
- Johnson & Johnson
- Leo Pharma A/S
- Merck KGaA
- Nestlé S.A
- Novartis AG
- Pfizer Inc
- Curatio Healthcare
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 125 |
| Published | April 2024 |
| Forecast Period | 2022 - 2029 |
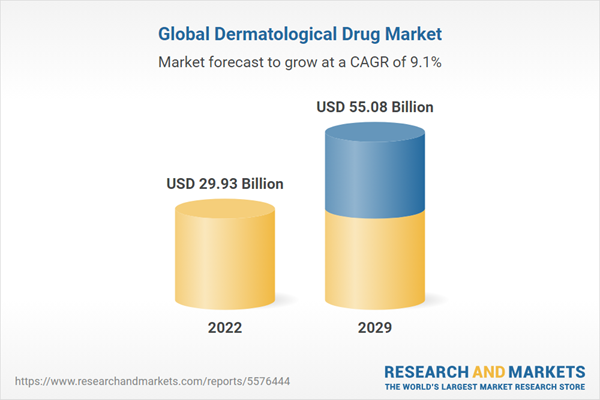
| Estimated Market Value ( USD | $ 29.93 Billion |
| Forecasted Market Value ( USD | $ 55.08 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |