Safety needles are advanced needs having the feature of an inbuilt safety mechanism that can avoid needle-linked injuries to the patients. The prime reason that is expected to drive the market growth during the forecasted period is the growing advent of diseases and rising vaccination in an attempt to avoid chronic diseases. With rising complexities in lifestyle and the growing prevalence of chronic diseases, expenditure on diagnosis, treatments, and vaccinations has surged significantly, which has increased the market for safe needles.
Furthermore, the demand for single-use safety needles for better prevention against potential infection of unwanted pathogens entering the body has provided a new opportunity for the industry’s expansion. Moreover, an increase in the geriatric population and demand for safe needles for better body suitability also drive the market growth. Robust investment by the government in thehealthcaresector is also projected to provide stable growth.
Geographically, the global safety needles are divided into North America, South America, Europe, the Middle East and Africa, and the Asia Pacific regions for the study. The Asia Pacific and North American regions are expected to hold a significant market share. The Asia Pacific region has been emerging as a medical hub. Compared to their countries, Americans and the European population find medical treatment in this region more cost-effective and cheaper. An average American can save up to 90% of the expenditure on healthcare if the patient gets treatment from the Asia Pacific region. Furthermore, rising healthcare infrastructure and facilities in the region will further expand themedical tourism marketand provide substantial growth for safety needles.
The European region has observed a high rise in the proportion of the geriatric population and a significant increase in chronic, especially cardiovascular disease, which is projected to create the demand for safe syringes for diagnosis, treatment, and medicine intake.
Market Drivers:
Rising healthcare expenditure and instances of diseases are expected to increase the market.
One of the prime reasons driving the market growth of safety needles is rising healthcare expenditure and instances of diseases. With growing complexities in lifestyle, a surge in cases of cardiovascular diseases has increased. Diagnosis, treatment, and medicine intake have increased significantly, which has raised safety needle adaption. Data from the World Health Organization (WHO) shows that in 2019, 17.9 patients lost their lives owing to cardiovascular illnesses, which accounted for 32% of deaths in the year. 6.460 million deaths among patients under 65 years of age were caused by CVDs.Rising instances of chronic diseases have significantly increased health expenditure for better healthcare. Data from the World Bank shows that per capita health expenditure has increased globally from US$999.104 in 2015 to US$1,111.082 in 2018. Increasing health expenditure and treatment are expected to surge the market for safety needles.
Furthermore, international organizations have also taken the initiative to reduce the burden of chronic diseases through awareness programs, medication, and treatments. WHO aims to reduce the global prevalence of blood pressure issues by 25% from 2010 to 2025. WHO declared that 50% of the beneficiaries under this program will receive continuous drug therapy and other treatments to prevent CVD-linked death by 2025. Promising prevention and treatment prospects are expected to increase market opportunities.
Market Restraint:
Rising syringe and needle waste create an environmental threat that could hamper the market.
A major problem faced by the safety needle industry is the disposal of needles. Safety syringes are predominantly single-use syringes, and their improper disposal creates syringes and needles pollution. With the rising usage of needles and syringes, a surge in syringe pollution has been observed worldwide. In March 2021, San Francisco collected more than 13,000 syringes and needles waste, an increase of 10 times than a decade ago. Furthermore, improper disposal of needles raises the chances of spreading diseases such as HIV and hepatitis. The surging needles market is expected to increase further pollution, which degrades the environment and hence has raised concerns.Key Developments:
- January 2023-MTD Group received FDA clearance for its DropSafeTM SicuraTM passive safety needle for intramuscular and subcutaneous injection of vaccines and drugs. The needle is designed to eliminate needlestick injuries, providing a fully safe injection experience. The needle is protected by a transparent shield that automatically locks after injection, requiring no incremental activation step by the healthcare professional (HCP). The safety of HCPs always been a top priority for MTD Group, and the DropSafeTM SicuraTM is going to be available to pharmacy chains and other healthcare practices in the US.
- January 2023-Milestone Scientific partnered with Sweden & Martina to distribute its STA Single Tooth Anesthesia System® in Spain, Portugal, and France. The agreement grants Sweden & Martina exclusive rights to market STA in these markets, replacing its current distributor in Italy. The STA system, which uses precise, digitized, and computer-controlled dosing, offers painless injections, shorter wait times, fewer complications, and reduced patient cancellations. The partnership aims to grow dentists' business and improve patient experience.
- November 2022-Neogen Corporation introduced a new dual-detecting needle for production farmers, enhancing the effectiveness of its Ideal® D3™ detectable needles. The patent-pending D3X needles feature Neogen's metal alloy needles with stronger side walls, increasing load strength and reducing the risk of bending and breaking. They also feature a highly visible red extraction collar to prevent needles from submerging in animal skin. The new needles offer increased visibility, quick removal, and increased efficiency, ensuring food safety and preventing needles from reaching consumers.
- March 2022-NuGen Medical Devices signed a 5-year distribution agreement with Harrington Consultants Limited and Al Mufid Pharmaceuticals for the distribution of its needle-free injection device, InsuJet™. The agreement cover Kuwait for at least CA$ 2,000,000, with regulatory approval from Kuwait Health Authorities. Kuwait ranks third globally for adult diabetes prevalence at 24.9%. InsuJet™ is the first self-administered needle-free injection system approved by Health Canada, providing safe and cost-effective drug delivery for millions of patients suffering from diabetes or other chronic illnesses.
Key Market Segments:
By Type
- Active Safety Needles
- Passive Safety Needles
By Route Of Administration
- Subcutaneous
- Intravenous
- Others
By End Users
- Hospitals and clinics
- Ambulatory care centers
- Diagnostic centers
- Others
By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- United Kingdom
- Germany
- Italy
- France
- Others
- Middle East and Africa
- UAE
- Saudi Arabia
- Others
- Asia Pacific
- Japan
- China
- India
- South Korea
- Taiwan
- Thailand
- Indonesia
- Others
Table of Contents
Companies Mentioned
- BD
- Cardinal Health
- B. Braun Melsungen Ag
- Medline Industries, Inc.
- Johnson & Johnson Services, Inc.
- Nipro Medical Corporation
- Smith’s Group Plc
- Métier Medical Limited
- Olympus Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 117 |
| Published | April 2024 |
| Forecast Period | 2022 - 2029 |
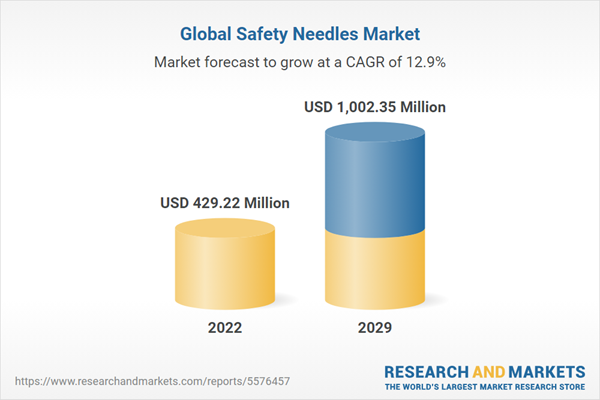
| Estimated Market Value ( USD | $ 429.22 Million |
| Forecasted Market Value ( USD | $ 1002.35 Million |
| Compound Annual Growth Rate | 12.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |