COVID-19 impacted the global supply chain of pharmaceuticals, affecting the acute bacterial skin and skin structure infection (ABSSSI) market. In the early phases of the pandemic, the market saw a significant sales decline due to stringent lockdowns, supply chain restrictions, and manufacturing halts. However, according to the study published in Acta Medica Indonesia in January 2021, bacterial skin and skin structure infections are worsened in COVID-19 patients and lead to prolonged hospitalization, thereby boosting the market. According to a study published in the Journal of Infectious Diseases in Clinical Practice, many high-risk COVID-19 individuals are also more likely to experience severe bacterial infections, frequently requiring hospitalization. Thus, during the initial stages of the pandemic, there was a slight decline in the market's growth. The high risk of ABSSSI in COVID-19 patients led to increased adoption of therapeutics.
The rising prevalence and awareness of acute bacterial skin and skin structure infection, as well as increased R&D activities by pharmaceutical and biopharmaceutical companies to supply new products and novel treatments, contribute to the market's growth.
Two of the most common bacteria that cause acute bacterial skin and skin structure infections are Streptococcus pyogenes and Staphylococcus aureus, including methicillin-resistant S. aureus. Gram-negative bacteria, other Streptococcus species, and Enterococcus faecalis are less common causes. According to a study published in the Infection and Drug Resistance Journal in April 2022, a crucial emerging concept in the treatment of acute bacterial skin and skin structure infections was the availability of various approved agents with anti-MRSA activity. The availability of anti-MRSA drugs in the management of ABSSSI is expected to drive the market.
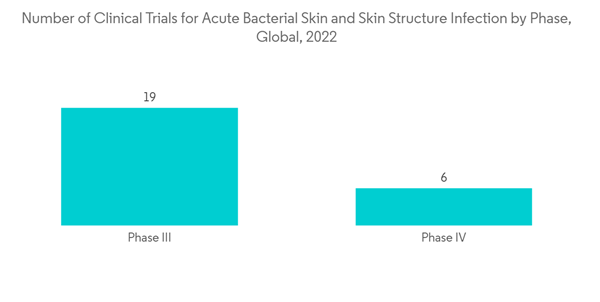
However, growing pipeline development activity and rising R&D expenditure by pharmaceutical and biotechnology companies, along with the approval of drugs, are anticipated to drive the market over the forecast period. For instance, as per clinicaltrials.gov, in May 2021, Ceftobiprole Medocarial under Phase III trials proved an effective treatment option for acute bacterial skin and skin structure infections. China National Medical Products Administration (NMPA) approved Zai Lab Ltd's new drug application for NUZYRA (omadacycline), a novel antibiotic with both oral and intravenous (IV) formulations, for the treatment of community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSSI).
Growing product launches and various activities by key market players, such as mergers and acquisitions, collaborations, and partnerships, are anticipated to drive the market. For instance, in July 2021, Melinta Therapeutics launched KIMYRSA (oritavancin), a lipoglycopeptide antibiotic that delivers a complete course of therapy for acute bacterial skin and skin structure infections (ABSSSI) in a single, one hour, 1,200 mg infusion.
Thus, the rising prevalence of ABSSSI, increasing strategies by key players, and rising R&D of therapeutics of ABSSSI are expected to boost the market over the forecast period. However, stringent regulatory policies by government bodies and patent expiration are expected to hinder the market in the future.
Key Market Trends
Hospital-acquired ABSSI is Expected to Hold Significant Market Share During the Forecast Period
Infections acquired in a healthcare setting are known as hospital-associated infections (HCAIs). They may cause (or prolong) stays in acute care hospitals, increase medical and economic burdens, and cause morbidity and mortality. According to a study published in the Journal of Antimicrobial Chemotherapy in November 2021, adult inpatients in the National Health Service hospitals in England have 834,000 hospital-acquired infections (HCAIs) each year, accounting for 7.1 million occupied hospital bed days (21% of total annual bed days) and a EUR 2.7 billion (USD 2.8 billion) economic burden. Such a huge burden of hospital-acquired infections is expected to drive the segment's growth due to the rising adoption of ABSSSI therapies for these infections.According to the same source, the prevalence of HCAI is estimated to be 6.0%. The most common infections were skin and soft tissue infections (SSTIs), which included surgical site infections (SSIs). Patients in intensive care units had the highest rate of hospital-acquired ABSSSI. Thus, growing prevalence of hospital-acquired infection is expected to boost the segment.
According to a study published in the International Journal of Antimicrobial Agents in August 2022, almost 10% of all hospital admissions in the United States and around 15% of all infections treated in European hospitals are due to ABSSSIs. Such a huge prevalence of hospital-based ABSSSI is expected to drive the segment due to the increased adoption of treatments for ABSSSI.
Therefore, as the high burden of hospital-acquired ABSSSI is expected to drive the market over the forecast period
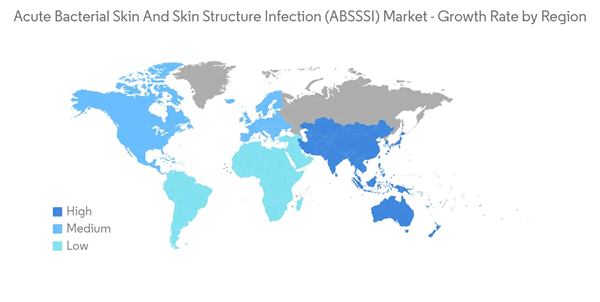
North America Holds a Significant Share in the Market and is Expected to Continue the Same During the Forecast Period
North America may continue to hold a significant share in the acute bacterial skin and skin structure infection market during the forecast period due to extensive R&D activity, R&D spending, product pipeline, and novel product launches.The growing product pipeline and product approval by regulatory authorities are expected to boost the market over the forecast period. For instance, in July 2021, the US FDA approved AbbVie's DALVANCE (dalbavancin) to treat acute bacterial skin and skin structure infections (ABSSSI) in pediatric patients from birth. DALVANCE is a single dose option administered as a 30-minute intravenous (IV) infusion for the treatment of ABSSSI caused by designated susceptible Gram-positive bacteria in pediatric patients, including infections caused by methicillin-resistant Staphylococcus aureus (MRSA). Such launches are expected to drive the market in the region.
In January 2022, Paladin Labs Inc., a subsidiary of Endo International PLC, launched Xydalba (dalbavancin for injection), a 30-minute intravenous (IV) therapy in Canada for acute bacterial skin and skin structure infections (ABSSSI) that can be administered as a single- or two-dose regimen.
Thus, all such factors are expected to boost the market in North America over the forecast period.
Competitive Landscape
The acute bacterial skin and skin structure infection market is moderately competitive. The market comprises many small and large market players. However, with technological advancements and product innovations, mid-size to smaller companies are increasing their market presence by introducing new products at lower prices. Some of the market players are Glenmark Pharmaceuticals Ltd, Melinta Therapeutics Inc., Sandoz Inc. (a subsidiary of Novartis), Paratek Pharmaceuticals Inc., AbbVie Inc. (Allergan PLC), Merck & Co. Inc., Pfizer Inc., Melinta Therapeutics Inc.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Paratek Pharmaceuticals Inc.
- Melinta Therapeutics, Inc.
- Allergan PLC
- Merck & Co. Inc.
- Endo Pharmaceuticals Inc.
- Nabriva Therapeutics PLC
- Basilea Pharmaceutica Ltd
- Cipher Pharmaceuticals
- Menarini Group
- Sandoz Inc. (Novartis)
- Glenmark Pharmaceuticals Ltd
- Pfizer Inc.