The outbreak of COVID-19 showed an impact on the vertigo treatment market because healthcare services were significantly reduced due to social distancing measures taken by governments across the globe. For instance, according to a study published in the Ear Nose and Throat Journal in April 2021, the total number of vertigo or dizziness outpatients decreased significantly during the early pandemic. Declining patient visits to the hospital during COVID-19 hampered the vertigo treatment market. However, as the pandemic has subsided, the studied market is expected to have stable growth during the forecast period of the study.
Factors such as an increase in the incidence and prevalence of peripheral etiologies of vertigo, an increase in the geriatric population and healthcare expenditure, and higher disposable income are likely to drive the worldwide vertigo market forward. For instance, according to a study updated by the NCBI in January 2022, vertigo has a lifetime prevalence of 2.4% in the adult European population and it was also shown to be more common in women than men, with 3.2% versus 1.6%, respectively. With the rising prevalence of vertigo, the demand for its treatment is expected to increase and boost the market's growth.
Furthermore, vertigo may intensify with age, and elderly people account for most cases reported to doctors. According to a study published in BioMed Central (BMC) Geriatric Journal in February 2022, vertigo affects roughly 30% of adults over the age of 65 and over 50% of those over the age of 85. The prevalence of vertigo among nursing home patients has increased to about 45% (peaking between 80 and 90 years of age). Thus, as the geriatric population is expected to rise; the demand for vertigo treatment is also anticipated to rise, which is expected to drive the market over the forecast period.
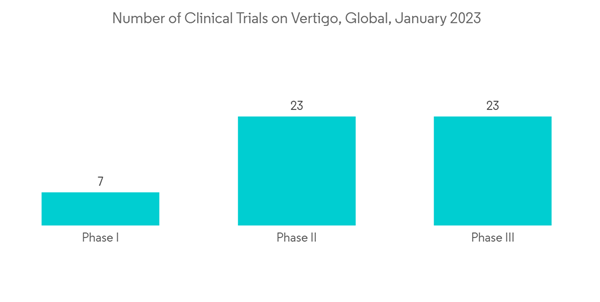
Key companies have employed strategies to gain a presence in this market, including new product launches, expansions, agreements, joint ventures, partnerships, and acquisitions. For instance, in July 2022, Sound Pharmaceuticals enrolled the first patient in its phase 3 clinical trial involving SPI-1005 for the treatment of Meniere's Disease (STOPMD-3).
Ignorance of the symptoms and causes of vertigo restrains the market over the forecast period.
Vertigo Treatment Market Trends
Peripheral Vertigo Segment is Expected to Hold Significant Market Share Over the Forecast Period
Peripheral vertigo occurs due to a problem in the part of the inner ear that controls body balance. These inner ear areas are called the vestibular labyrinth or semicircular canals. Sometimes peripheral vertigo may also occur due to a problem in the vestibular nerve, which is the nerve between the inner ear and the brain stem. Peripheral vertigo is most often caused by a benign process, with benign paroxysmal positional vertigo (BPPV) being the most prevalent cause, and the rising prevalence of it is anticipated to drive the segment over the forecast period.According to a study published in the Journal of Neurology in November 2021, benign paroxysmal positional vertigo (BPPV) is the most common cause of dizziness/vertigo worldwide, accounting for 24.1% of all hospital visits for dizziness/vertigo, with a lifetime incidence of 2.4%. Recurrences of BPPV are common, with a recurrence rate of 15-20% per year. Furthermore, according to an article published by PubMed Central in August 2021, peripheral vertigo is considered most common in benign paroxysmal positional vertigo, followed by Ménière disease and vestibular neuritis.
Furthermore, according to ClinicalTrials.gov updates on April 2022, Auris Medical AG is conducting phase II trials to evaluate AM-125 in the treatment of acute peripheral vertigo. The increasing clinical trials on peripheral vertigo by key market players are also anticipated to drive the segment growth.
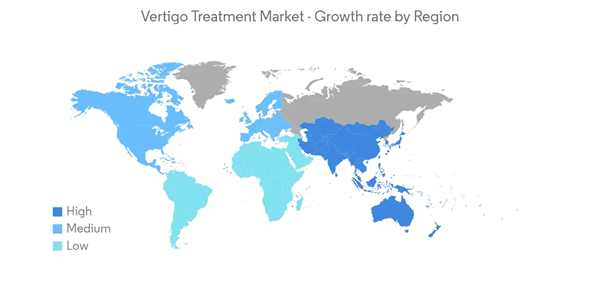
North America is Expected to Hold a Significant Share in the Market Over the Forecast Period
North America is expected to hold a significant share of the market due to a rise in the prevalence of vertigo, a growth in the elderly community, and a demand for ongoing innovation. The United States accounted for the maximum share in the region.According to the article published in the National Library of Medicine in February 2022, benign paroxysmal positional vertigo has an annual incidence of 64 per 100,000 in the United States. Furthermore, with each decade, this number increases by 38%, equating to over 200,000 new cases in the United States annually.
Additionally, the rising geriatric population is one of the contributing factors to the growth of the market in the region, as vertigo is often associated with older age. According to data published by Statistics Canada in July 2022, it is estimated that around 7,330,605 people are aged 65 years or older in Canada, and this accounts for 18.8% of the total population.
Key firms have employed various techniques to gain a presence in this market, including new product launches, expansions, agreements, joint ventures, partnerships, and acquisitions. For instance, in July 2022, Sound Pharma initiated enrollment in a pivotal phase 3 clinical trial of SPI-1005 for the treatment of hearing loss and tinnitus in Meniere’s disease associated with vertigo.
Vertigo Treatment Industry Overview
The vertigo treatment market is highly fragmented, and the major players have used several strategies to increase their foothold in this market. Some market players are AdvaCare Pharma Amneal Pharmaceuticals LLC, Lupin, Epic Pharma, LLC, Pfizer Inc, and Teva Pharmaceutical Industries Ltd, among others.Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Viatris Inc
- Amneal Pharmaceuticals LLC
- Epic Pharma, LLC
- Pfizer Inc
- Teva Pharmaceutical Industries Ltd,
- Zydus Cadila
- Sun Pharmaceutical Industries Limited
- Cipla Inc.
- RPG Life Sciences Limited
- Prestige Consumer Healthcare Inc.
- AdvaCare Pharma
- Lupin