Monitoring the neurophysiological activities of the central and peripheral nerve systems while undergoing surgery is known as neuromonitoring. Consequently, is anticipated to drive the market share of neuromonitoring devices. Technology has improved substantially in the medical industry, and newer, more sophisticated tools are being created to support doctors in their daily activities. In recent years, many of these gadgets have evolved into noninvasive or less invasive ones. Because of the magnitude of the neuromonitoring device industry, it is now possible to extract a wealth of information from multimodal noninvasive monitoring that can be used for both therapeutic and predictive purposes.
To evaluate the functional capacity of the brain, cerebellum, spinal cord, or peripheral and cranial nerves during surgery, electrophysiologic monitoring, also known as organization can measure, is utilized. The purpose of monitoring is to warn the surgeons and anesthetist of potential harm so that care can be changed in time to avoid lasting harm. Areas of the nervous system can sometimes be mapped using neuromonitoring in order to direct procedural management. Electroencephalograms and spontaneous electromyograms, as well as evoked responses to stimuli, can also be recorded as part of neuromonitoring such as motor evoked potentials, somatosensory evoked potentials, triggered electromyography, and brainstem auditory evoked potentials. Multiple techniques are frequently combined to improve the usefulness of monitoring and get around the shortcomings of individual procedures.
In many surgical procedures, neuromonitoring has replaced intraoperative wake-up testing as a routine practice. A specialist team with in-depth knowledge of the methods employed performs neuromonitoring. In most cases, there is no 'standard of care' for intraoperative neuromonitoring, and the surgeon and monitoring team choose procedures to evaluate or safeguard tissues at risk.
As of September 2019, the WHO estimates that 50 million people suffer from dementia, with around 10 million new cases being diagnosed each year. This is one of the key reasons that older people are disabled or dependent on others all around the world. In June 2019, the WHO estimated that 50 million individuals suffered from epilepsy, with over 80 percent of them residing in low- and middle-income nations. According to estimates, 150,000 Americans, or 48 out of every 100,000, will suffer from epilepsy each year. In the United States, 3.4 million people suffer from epilepsy.
COVID-19 Impact Analysis
The market for neuromonitoring equipment suffered a significant setback during the pandemic because there was less demand in hospitals and clinics, even if the healthcare industry saw good growth throughout the period. The hospital authorities limited the admission of patients with various disorders, and this factor has lessened the demand for neuromonitoring devices. The coronavirus is highly contagious and spreads quickly in the environment, infecting many people in the shortest amount of time. Additionally, a lot of neurosurgeries were delayed due to the increase in coronavirus patients. This aspect is also expected to hinder market expansion during the pandemic.Market Growth Factors:
Increase in the Use of Neuromonitoring Technology
The market revenue for neuromonitoring devices is also anticipated to rise due to an increased prevalence of hospitalizations, emergency room visits, and admissions due to significant medical illnesses and brain injuries from car accidents. Additionally, the frequency of catastrophic accidents and neurological diseases is increasing daily. According to UN estimates from 2007, over 1 billion people, or 1 in every five or six people, were affected by neurological conditions such as seizures, strokes, Parkinson's, multiple sclerosis, and headaches. According to the Centers for Disease Control and Prevention, 2.5 million traumatic accidents take place annually in the United States and cause brain infections, meningitis, and aneurysms.Increasing Clinical Applications and Funding for Research and Development
Spinal cord stimulation is one of the most widely used therapies for chronic back pain because of the structural and functional abilities of spinal cord nerves to manage pain feeling. Treatment for neurological conditions like epilepsy most frequently involves vagus nerve stimulation. Although these devices have well-established treatment algorithms, numerous academic institutions and business leaders are running clinical trials to ascertain whether they are effective in treating other diseases. For example, Huashan Hospital in Beijing, China, is conducting experiments to see whether spinal cord stimulation may be employed to treat awareness issues (DOC).Marketing Restraining Factor:
Neuromonitoring devices are extremely expensive
One of the main factors restricting the market for brain monitoring is its high cost, especially in developing nations with lax reimbursement regulations. Devices for monitoring the brain are quite complicated, and the procedures required to use them are generally expensive. For instance, an EEG typically costs between USD 200 and USD 700; however, continued monitoring might increase the price to USD 3,000. An ICP monitor is about USD 340-500, while a dual-channel TCD is roughly USD 3,200-3,200. Similar to that, a single MEG system prices between USD 2.5 and 3 million. Healthcare facilities find it difficult to obtain MEG devices due to the expensive cost, even in nations where high-tech medical equipment is extensively used.Product Outlook
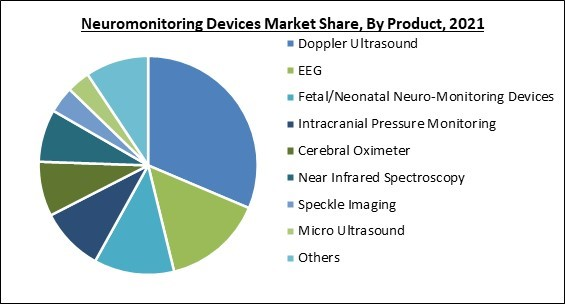
Based on Product, the market is segmented into Doppler Ultrasound, EEG, Fetal/Neonatal Neuro-Monitoring Devices, Intracranial Pressure Monitoring, Cerebral Ox meter, Near Infrared Spectroscopy, Speckle Imaging, Micro Ultrasound, and Others. The Doppler Ultrasound segment acquired the highest revenue revenue share in the Neuromonitoring Device Market in 2021. Doppler ultrasounds use sound waves to produce images of the body's veins and arteries showing how blood flows across them. The goal is frequently to examine blood flow in the legs and arms. During a Doppler ultrasonography, a portable device emits sound waves that reflect off moving objects like blood cells.Regional Outlook
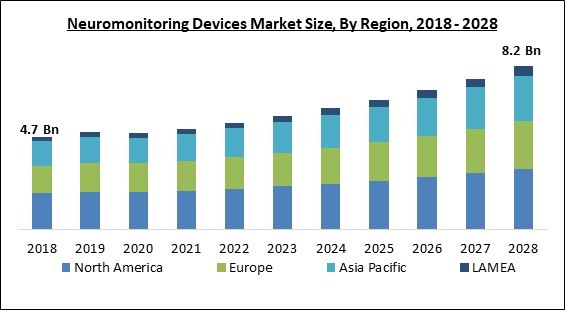
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. The North America segment procured the largest revenue share in the Neuromonitoring Device Market in 2021. This is due to the patients' growing demand for higher-quality healthcare facilities with more modern technology. The market revenue in North America may also rise further with the implementation of new and cutting-edge technologies. In response to the increased demand for neuromonitoring devices and the increasing incidence of neurological illnesses like epilepsy, major market participants have concentrated on releasing new products in the market.The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include General Electric (GE) Co. (GE Healthcare), Koninklijke Philips N.V., Medtronic PLC, Nihon Kohden Corporation, Natus Medical Incorporated, Nuvasive, Inc., Advanced Brain Monitoring, Inc., Intranerve Neuroscience Holdings, Llc, Specialtycare, and RIMED Ltd.
Recent Strategies Deployed in Neuromonitoring Devices Market
- Apr-2022: GE Healthcare joined hands with Medtronic, a global leader in medical technology, services, and solutions. This collaboration focused on providing unusual requirements and needs for maintenance at Office-Based Labs and Ambulatory Surgery Centers. Moreover, consumers can access comprehensive product offerings, economic solutions, and superior benefits.
- Nov-2021: Royal Philips completed the acquisition of Cardiologs, a France-based medical technology company. With this acquisition, Cardiologs is expected to bolster Philips’ cardiac diagnostics and monitoring portfolio with creative software technology, electrocardiogram examination, and reporting assistance.
- Aug-2021: NuVasive received a US Food and Drug Administration approval along with the commercial launch of the Pulse platform for spine surgeries. The platform is a combined technology medium developed to improve the capability, safety, and procedural duplicability of spine surgery. In addition, the Pulse allows doctors to efficiently access numerous technologies from a consolidated footprint as well as discourse various typical surgical provocation.
- Feb-2021: Royal Philips took over BioTelemetry, a leading U.S.-based supplier of remote cardiac monitoring and diagnostics. Under this acquisition, BioTelemetry is a substantial entrance with Philips’ cardiac care offering to transform the delivery of care within the health continuity with combined solutions.
- Oct-2020: Medtronic received a U.S. Food and Drug Administration approval for NIM Vital nerve monitoring system. The NIM Vital allows doctors to recognize, ensure, and observe nerve function to assist decrease the chance of nerve damage during the neck and head surgery. In Addition, NIM Vital system utilizes exclusive technology to deliver precise intraoperative nerve essential data that report surgical strategy, improve operative accuracy and efficiency and assists rescue patients' life.
- Feb-2020: Medtronic acquired Digital Surgery, a UK-based firm with a focus on surgical data, artificial intelligence, and analytics. Through this acquisition, Medtronic aimed to reinforce its offering and its robotic-assisted surgery forum.
- Nov-2019: SpecialtyCare completed the acquisition of Synapse, an Indianapolis-based Intraoperative Neuromonitoring provider. This acquisition is expected to extend SpecialtyCare’s industry leadership standing as the biggest and most complete supplier of patient-based IONM services to hospitals, surgeons, health systems, and surgery centers.
- Apr-2019: SpecialtyCare took over Neuropath, Atlanta-based IONM supplier. This acquisition is expected to extend SpecialtyCare’s industry supervision status as the biggest and most wide supplier of patient-based outsourced IONM assistance to hospitals, doctors, health systems, and surgery centers.
- Dec-2018: SpecialtyCare acquired Precedent Health, Intraoperative neuromonitoring provider. This acquisition is expected to extend SpecialtyCare’s industry supervision status as the biggest and most wide supplier of patient-based outsourced IONM assistance to hospitals, doctors, health systems, and surgery centers.
- Aug-2018: NuVasive came into a partnership with Siemens, a global powerhouse focusing on the areas of electrification, automation, and digitalization. Through this partnership, the companies aimed to enhance visualization of the anatomy during spine implant procedures. Moreover, the partnership is expected to combine NuVasive’s new Pulse surgical automation platform within Siemens Healthineers’ mobile 3D imaging for enhanced quality guarantee during surgery.
Scope of the Study
Market Segments Covered in the Report:
By Product
- Doppler Ultrasound
- EEG
- Fetal/Neonatal Neuro-Monitoring Devices
- Intracranial Pressure Monitoring
- Cerebral Oximeter
- Near Infrared Spectroscopy
- Speckle Imaging
- Micro Ultrasound
- Others
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- General Electric (GE) Co. (GE Healthcare)
- Koninklijke Philips N.V.
- Medtronic PLC
- Nihon Kohden Corporation
- Natus Medical Incorporated
- Nuvasive, Inc.
- Advanced Brain Monitoring, Inc.
- Intranerve Neuroscience Holdings, Llc
- Specialtycare
- RIMED Ltd.
Unique Offerings from the Publisher
- Exhaustive coverage
- The highest number of market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- General Electric (GE) Co. (GE Healthcare)
- Koninklijke Philips N.V.
- Medtronic PLC
- Nihon Kohden Corporation
- Natus Medical Incorporated
- Nuvasive, Inc.
- Advanced Brain Monitoring, Inc.
- Intranerve Neuroscience Holdings, Llc
- Specialtycare
- RIMED Ltd.