Apheresis is the removal of blood from a donor's body, the expulsion of one or more blood products such as platelets, white blood cells, or plasma, and the fluid replacement of the surviving blood back into the donor during or after the procedure. In apheresis, whole blood from a patient or donor's body is converted within an instrument that functions essentially as a centrifuge, separating the components of whole blood. The solid residues are then re-transfused into the donor after one of the divided portions is removed.
Red blood cells, white blood cells, platelets, and plasma are the four components of human blood. To cure a disease, one of those elements may need to be removed or supplanted using a procedure known as apheresis. Apheresis makes use of a centrifuge to separate blood into its constituents based on density.
The increased use of plasma donation in the treatment of burn sufferers, trauma patients, and patients dealing with severe disorders or major injuries contributes to the market's growth. Furthermore, patients admitted due to COVID-19 receive plasma treatment therapy (convalescent plasma). On the other hand, progressions in the healthcare sector, emerging markets are expected to provide significant lucrative apheresis potential market for key players.
Haemoneticsis, a blood management product company, received FDA (Food and Drug Administration) approval in October 2020 for NexSys PCS with persona technology, which personalizes plasma gathering based on an individual donor's body structure. Furthermore, Kaneka Corporation, a chemical, food, and medical device company is expected to then launch rheocarna, an adsorption type blood purifying device that treats a serious case of arteriosclerosis obliterans, in October 2020.
COVID-19 Impact Analysis
Due to the COVID-19, blood clotting variables including platelet count, D-dimmer, fibrinogen, prothrombin time (PT), and activated partial thromboplastin time (aPTT) were discovered as complications in patients who were severely infected, resulting in an extensive use of plasma therapy treatment. The US FDA has approved emergency use authorization (EUA) of convalescent plasma (CCP) therapy in hospitalized patients with COVID-19 in August 2020. Terumo BCT Inc. Terumo BCT's Spectra Optia Apheresis System, in combination with Marker Therapeutics' D2000 Adsorption Cartridge, got FDA approval in April 2020 for the Emergency Use Authorization (EUA) to treat persons aged 18 years or older with confirmed COVID-19 disease.Market Growth Factors
Apheresis donors are essential element
When a person has a regular routine, they do not want to change it because it is most advantageous for them. In reality, apheresis donations may be more important at times when specific component needs are greater. Contrary to popular belief, double red cell campaign contributions can be more useful in some cases. Depending on the program, there are prerequisites for this type of donation, in which male and female donors must fulfill more specific physical standards to donate. Although this type of donation can take up to a half-hour longer, it has the advantage of being the most commonly used part of the blood. The use of red blood cells during surgery and trauma is widespread.Increasing investments in healthcare technology
With the pandemic wreaking havoc around the world, the health-care industry has seized the opportunity to innovate and improve technologically. Investors' desire to invest in the healthcare industry. In the midst of a pandemic, counseling, telehealth, monitoring patients, and even digital medical insurance have become increasingly popular. Companies that previously placed a premium policy on employees' health during new employee onboarding are now recognizing the value of having a healthy workforce. When it comes to telemedicine, there are numerous benefits. RFID solutions, pulse, and ECG are just a few examples of devices that can monitor a patient's vital signs in real-time.Market Restraining Factors
Apheresis Donation Complications
As the person sits in a comfortable seat, the procedure usually takes about two to four hours, depending on how ill a patient is and what needs to be collected. Although apheresis is usually painless, some adverse effects may occur, including a dip in blood pressure, lightheadedness, perspiration, or soreness in the arm where the needle is inserted. Since apheresis takes longer than conventional blood donation and uses needles in both arms instead of just one, there's a higher likelihood of such side effects. There is a minor risk of allergic reaction or fever if a patient receives donor elements such as red blood cells as part of their treatment, but patients are continuously watched and any adverse reactions are swiftly identified and managed.Procedure Outlook
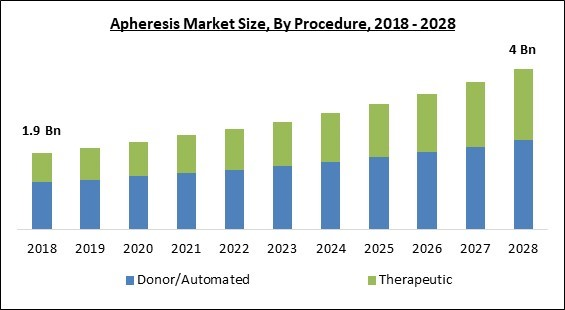
Based on Procedure, the market is segmented into Donor/Automated and Therapeutic. The donor/automated segment garnered the largest revenue share in the apheresis market 2021. It is due to an increase in apheresis platelet and apheresis plasma charitable contributions has contributed to market growth. The donor's blood is extracted, and the cell separator collects Platelets, Plasma, and/or Red Cells. The residual components, as well as saline fluid, are transferred to the donor. Each operation takes between one and two hours to complete.Product Outlook
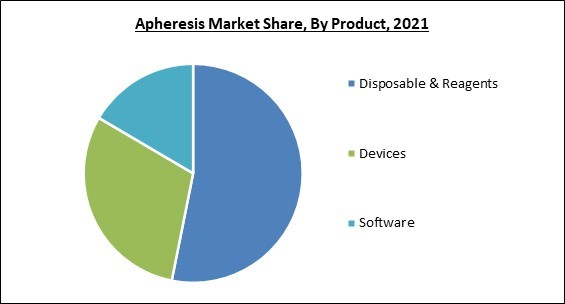
Based on Product, the market is segmented into Disposable & Reagents, Devices, and Software. The device segment witnessed a significant revenue share in the apheresis market 2021. It is due to the devices use in-line automated technology to separate whole blood into component fractions, with the desired fraction removed and the remaining components returned. In TPE, this separation can be accomplished through centrifugation or outer layer filtration using either constant or unreliable flow.Method Outlook
Based on Method, the market is segmented into Centrifugation, Membrane Separation, and Selective Adsorption. The centrifugation segment procured the largest revenue share in the apheresis market 2021. Due to the common use of centrifugation to obtain blood components, such as packed red blood cells (PRBCs) and fresh frozen plasma (FFP), in one heavy spin. The blood component divider draws whole blood from a patient and separates it into its constituent elements using centrifugal force as its operating principle.End User Outlook
Based on End User, the market is segmented into Blood Centers, Hospitals, and Others. The hospital segment witnessed a significant revenue share in the apheresis market 2021. Due the various advance facilities and professional working in the hospital propels the segment. When apheresis is performed on a patient, one of the separated components is removed when it is impacted by the disease, and the remaining variables are then re-transfused to the patient. Apheresis is used for a variety of hematological, neurologic, and transplant-related reasons.Component Outlook
Based on Component, the market is segmented into Plasma (Plasmapheresis), Platelets (Plateletpheresis), Leukocytes (Leukapheresis or leukopheresis), Lymphocytes (Lymphopheresis or lymphapheresis), and RBC's (Erythropheresis). The Plasma (Plasmapheresis) segment garnered the largest revenue share in the Apheresis market 2021. Plasmapheresis also known as plasma exchange, is a medical procedure in which whole blood is separated into cell components and plasma using a device. The plasma is discarded and supplanted with a colloid fluid, which is then combined with the cell functions and returned to the patient. Typically, the colloid fluid is a mixture of human serum albumin and/or fresh and frozen plasma.Cardinal Matrix-Apheresis Market Competition Analysis
Regional Outlook
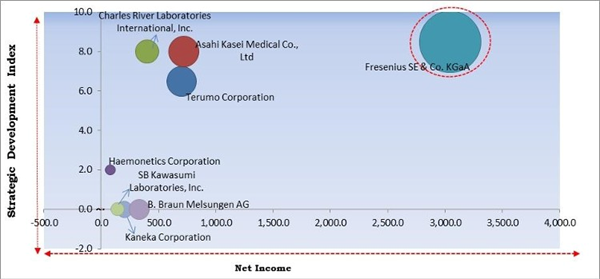
Based on Regions, the market is segmented into North America, Europe, Asia Pacific, and Latin America, Middle East & Africa. The North America region procured the largest revenue share in the apheresis market in 2021. Due to the early adoption of novel apheresis systems and the rise in the predominance of blood-related disorders in this region. This region's large share can be attributed to improved access to advanced blood collection innovations, the existence of developed healthcare architecture, and the strong influence of foremost apheresis firms in the country.The major strategies followed by the market participants are Acquisitions. Based on the Analysis presented in the Cardinal matrix; Fresenius SE & Co. KGaA are the forerunners in the Apheresis Market. Companies such as Charles River Laboratories International, Inc., Terumo Corporation and Asahi Kasei Medical Co., Ltd are some of the key innovators in the Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Haemonetics Corporation, Fresenius SE & Co. KGaA, Terumo Corporation, Asahi Kasei Medical Co., Ltd., Kaneka Corporation, Cerus Corporation, B. Braun Melsungen AG, Nikkiso Co., Ltd., Charles River Laboratories International, Inc., and SB Kawasumi Laboratories, Inc.
Strategies Deployed in Apheresis Market
Partnerships, Collaborations and Agreements:
- Nov-2021: Terumo Blood and Cell Technologies, a subsidiary of Terumo Corporation signed a co-commercialization agreement with Immunicom, an immuno-oncology company located in San Diego. Through this agreement, the companies aimed to integrate Terumo Blood and Cell Technologies' dominating Spectra Optia Apheresis System within Immunicom's CE labeled immuno-oncology LW-02 Column, for patients with developed obstinate triple-negative breast cancer.
- Oct-2021: Terumo Blood and Cell Technologies joined hands with BioCentriq, a clinical manufacturing facility for cell and gene therapies. This collaboration aimed to boost the approval of automated production to provide novel cell and gene therapies (CGT) to patients more rapidly and affordably. Additionally, Terumo Blood and Cell Technologies' automated solutions and services associated with BioCentriq's abilities is expected to meet consumers' product evolution track and allow a flexible approach for the future.
Acquisitions and Mergers:
- Jun-2022: Asahi Kasei Medical completed the acquisition of Bionova Scientific, a biologics CDMO focused on developing and manufacturing recombinant protein products. Under this acquisition, the company aimed to deliver biopharmaceutical enterprise with further additional importance and higher consumer fulfillment by utilizing its actual bioprocess materials, tools, and biosafety testing services industry along with Bionova Scientific’s biologics CDMO company.
- Mar-2022: Fresenius Kabi, a subsidiary of Fresenius acquired took over Ivenix, a medical technology dedicated to eliminating infusion-related patient harm. Through this acquisition, the Fresenius Kabi aimed to reinforce and utilize Fresenius Kabi's stability, to enhance the organization's growth industries in medical technology and biopharmaceuticals. Moreover, Fresenius includes next-era infusion treatment platform praise and bolsters actual infusion treatment providing to develop a premium offering.
- Aug-2020: Charles River Laboratories International completed the acquisition of Cellero, an exclusive supplier of essential cellular products and services to cell treatment developers and producers. Under this acquisition, Charles River aimed to expand its offering of high-quality, human-derived biomaterials and connected services by utilizing Cellero’s product portfolio and donor sites in the US.
- Jan-2020: Charles River Laboratories International acquired HemaCare Corporation, a global leader in the supply and customization of human-derived biological products. This acquisition is expected to support patients' initial drug research actions, and accomplish long-term development plans, and improve shareholder worth.
Geographical Expansions:
- May-2022: Terumo Blood and Cell Technologies, a subsidiary of Terumo Corporation expanded its geographical footprint by establishing a new manufacturing facility in Douglas County, Colorado. Through this expansion, the company aimed to grow its footprint and financial dedication to provide stable jobs while producing products that touch patients’ dwell every second of each day. Additionally, Terumo Corporation aimed to operate 24/7 with four manufacturing plants that are designed for productivity with favorably automated accuracy production.
- Jan-2020: Fresenius Kabi subsidiary of Fresenius SE expanded its geographical footprint by establishing a new production site in the Dominican Republic. Through this expansion, the company aimed to enlarge manufacturing tasks in the Dominican Republic and is expected to improve its capabilities to develop and supply essential products for apheresis and plasma collection for patients across the world.
Approvals and Trials:
- Oct-2022: Terumo Blood and Cell Technologies received a U.S. Food and Drug Administration approval for Rika, the new plasma collection system. Rika is expected to change plasma assemblage center procedures. Additionally, Terumo Blood and Cell Technologies acted across the donor and plasma center background, forming a completely new environment.
- Oct-2020: Haemonetics Corporation received a U.S. Food and Drug Administration approval for the NexSys PCS system with Persona technology. The new, patented Persona technology is the latest creation on the NexSys PCS system and it optimizes plasma grouping based on an personal donor's body design.
Scope of the Study
Market Segments Covered in the Report:
By Procedure
- Donor/Automated
- Therapeutic
- Disposable & Reagents
- Devices
- Software
- Centrifugation
- Membrane Separation
- Selective Adsorption
- Blood Centers
- Hospitals
- Others
- Plasma (Plasmapheresis)
- Platelets (Plateletpheresis)
- Leukocytes (Leukapheresis or leukopheresis)
- Lymphocytes (Lymphopheresis or lymphapheresis)
- RBC's (Erythropheresis)
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Haemonetics Corporation
- Fresenius SE & Co. KGaA
- Terumo Corporation
- Asahi Kasei Medical Co., Ltd
- Kaneka Corporation
- Cerus Corporation
- B.Braun Melsungen AG
- Nikkiso Co., Ltd
- Charles River Laboratories International, Inc.
- SB Kawasumi Laboratories, Inc.
Unique Offerings from the Publisher
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Haemonetics Corporation
- Fresenius SE & Co. KGaA
- Terumo Corporation
- Asahi Kasei Medical Co., Ltd
- Kaneka Corporation
- Cerus Corporation
- B. Braun Melsungen AG
- Nikkiso Co., Ltd
- Charles River Laboratories International, Inc.
- SB Kawasumi Laboratories, Inc.