The rising prevalence of cancer & other chronic diseases, extensive R&D efforts to develop novel therapies, and the presence of a strong pipeline of drugs under trial are the major factors boosting the market growth.
As per the American Cancer Society report, the population of cancer survivors in the US is expected to grow from ~16.9 million (since January 2019) to 22.1 million by January 2030 due to the rising aging population. Therefore, the demand for biologics such as monoclonal antibodies (mAbs) is high owing to the rising prevalence of cancer and other chronic diseases. Biologics play a key role in cancer treatment due to the presence of its principal components in many therapeutic regimens and the cost-effectiveness of treatment for patients.
Most licensed mAbs effectively treat noncommunicable diseases, including cancers and autoimmune diseases. Furthermore, successful immunotherapies involving more than 40 licensed mAbs are efficient for revolutionizing cancer treatment and have significantly improved patients' overall and long-term survival compared to traditional approaches such as chemotherapy.
According to the International AIDS Vaccine Initiative (IAVI) report, the development of mAbs is one of the fastest-growing segments of biomedical research. Further, more than 50 mAbs were licensed in the last six years, and in 2019, seven of the ten best-selling novel drugs for cancer and autoimmune diseases were mAbs. Further, the demand for mAbs has increased with the growing number of noncommunicable and infectious diseases for which mAbs are or might prove effective treatment. The sale of mAbs is predominant in the US, Canada, and Europe. Also, in terms of availability of mAbs in middle-income countries, India represents one of the most ideal countries, largely due to extensive biosimilar manufacturing capacity.
Moreover, several countries are taking strong initiatives to shorten the regulatory process for mAbs that are effective in the treatment of chronic diseases. For example, the Chinese Food and Drug Administration (CFDA), in 2017, announced a collaboration with the International Council for Harmonization to align regulatory processes relating to international standards, resulting in the approval of a number of mAbs, annually. In early 2019, the CFDA approved three mAbs from domestic developers and ten mAbs from multinational pharmaceutical companies. Such factors are stimulating the uptake of mAbs, which is contributing to the monoclonal antibody diagnostic reagent market growth.
Tests Insights
Based on tests, the monoclonal antibody diagnostic reagent market is segmented into double antigen sandwich chemiluminescence method, enzyme linked immunosorbent, assay recombinant immunoblot assay, and dot-immunogold filtration assay. The enzyme-linked immunosorbent assay segment is likely to hold the largest market share in 2022, and it is anticipated to register the highest CAGR during the forecast period. The rise in the number of chronic diseases increases the demand for the enzyme-linked immunosorbent assay (ELISA) tests for the diagnosis of hepatitis, AIDs, influenza, herpes simplex, chlamydia infections, and cancer, which is driving the growth of the enzyme-linked immunosorbent assay segment.Application-Based Insights
Based on application, the monoclonal antibody diagnostic reagent market is segmented into hormones diagnosis, tumor monitoring, virus detection, and others. The tumor monitoring segment is likely to hold the largest market share in 2022, and it is expected to register the highest CAGR from 2022 to 2028. Monoclonal antibody diagnostic reagents are widely used in the field of cancer diagnostics. The use of antibodies linked with fluorescent dyes or enzymes enables the detection of cancer and also determines the type of cancer. All these factors are driving the growth of the tumor monitoring segment.The National Center for Biotechnology Information (NCBI), Centers for Disease Control and Prevention (CDC), World Health Organization (WHO), Food and Drug Administration (FDA), American Society of Clinical Oncology (ASCO), Canadian Cancer Statistics, GTAI (Germany Trade and Invest), Health Ministry of Spain, Business Monitor International, National Institute of Population and Social Security Research Japan, Department of Health-Abu Dhabi, Globocan, Department of Emergency Medicine, and International AIDS Vaccine Initiative (IAVI) report are among the primary and secondary sources referred to while preparing the report on the monoclonal antibody diagnostic reagent market.
Table of Contents
Companies Mentioned
- BioGenex
- Bio-Rad Laboratories, Inc.
- Biocare Medical, LLC
- Celltrion Healthcare Co., Ltd.
- Creative Diagnostics
- GenWay Biotech
- Thermo Fisher Scientific Inc.
- ABclonal, Inc.
- Apto-Gen
- Abcam plc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 252 |
| Published | September 2022 |
| Forecast Period | 2022 - 2028 |
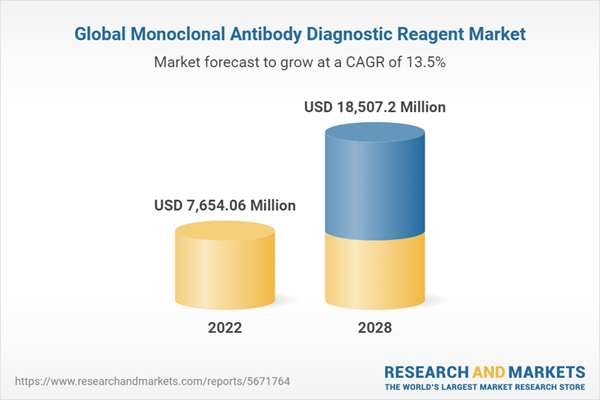
| Estimated Market Value ( USD | $ 7654.06 Million |
| Forecasted Market Value ( USD | $ 18507.2 Million |
| Compound Annual Growth Rate | 13.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |