A treatment called bariatric surgery involves operating on the digestive system primarily for weight-loss purposes. By reducing the number of meals that the stomach can consume or limiting the number of nutrients that are absorbed in the intestinal canal, bariatric surgery devices change the gastrointestinal tract. Numerous operations are being carried out for obese patients as a part of bariatric surgery.
The standard of care treatments for long-term weight loss (sleeve gastrectomy, Roux en-Y bypass, and biliopancreatic diversions with duodenal switch) primarily work by changing the levels of the gut hormones that regulate satiety and hunger, creating a new hormonal weight set point. In these operations, bariatric surgery is a hormonal surgery in which the shift in gut hormones occurs as a result of the restriction and malabsorption of the process.
COVID-19 Impact Analysis
The market for bariatric surgery devices has been severely damaged by the COVID-19 pandemic. Sales of bariatric surgery equipment in the medical equipment market witnessed a substantial decline in the initial stages of the pandemic due to the diffusion of COVID-19 infection. In addition, all elective surgeries were postponed or canceled during the pandemic to make the most of the available hospital space for COVID-19 patients. A large number of patients have been denied access to bariatric surgery due to the novel coronavirus's widespread distribution and the lockdown policies that followed it. With most doctors occupied with the uninterrupted care of corona patients during the ongoing pandemic, the only services available to patients in public hospitals were oncological therapies and surgical emergencies.Market Growth Factors
Rising Prevalence Of Obesity All Over The World
Formerly regarded as a last alternative for weight loss, bariatric surgery has gained a lot of popularity over time due to the fact that it is both safer and more efficient than conventional medical techniques, such as prescription drugs and dietary counseling. Bariatric surgery has increased steadily in recent years all over the world. The American Society for Metabolic and Bariatric Surgery (ASMBS) estimates that there were 158,000 bariatric procedures carried out in the US in 2011 as opposed to 198,851 in 2020. The market for bariatric surgery devices is predicted to develop as a consequence of the rising demand for related equipment & devices brought on by the rising number of performed bariatric procedures.Helps In Preventing A Number Of Fatal Disorders
Obesity brings on a number of other diseases with it, including diabetes, liver cirrhosis, and cardiovascular disorders. According to the Cleveland Clinic, type 2 diabetes can go into long-term remission after bariatric surgery. According to the study's findings, the technique is very successful in treating type 2 diabetes in obese people because it almost always leads to a three-year absence from insulin as well as other related medications. A person's risk of coronary heart disease, peripheral heart disorder, and stroke is considerably lowered post weight loss surgery. This factor is stimulating the growth of the bariatric surgery market.Market Restraining Factors
Complex Go-To-Market Procedures Along With The Dearth Of Skilled Personnel
One of the biggest factors, which is restricting the growth of the market is the increasing number of complications in the process of receiving approvals for the clinical use of these devices. Developing markets are working hard to establish a regulatory framework that will discriminate between pharmaceutical products and medicinal devices and equipment. The approval process is currently unstable and causes delays because there is no definite line between pharmaceuticals and devices. Therefore, it is anticipated that unstable regulatory frameworks will lengthen the clearance process for surgical equipment, somewhat restricting the market's expansion.Devices Type Outlook
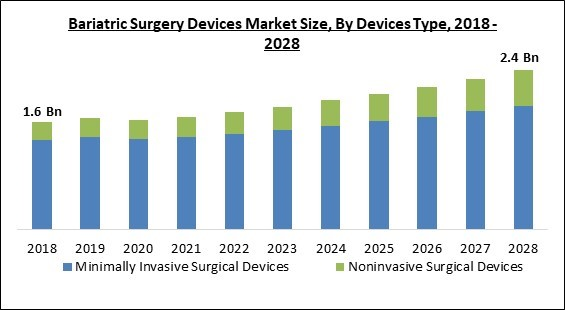
On the basis of Devices Type, the Bariatric Surgery Devices Market is bifurcated into Minimally Invasive Surgical Devices (Stapling Devices, Energy/Vessel-sealing Devices, Suturing Devices, and Accessories) and Noninvasive Surgical Devices. In 2021, the noninvasive surgical devices segment garnered a substantial revenue share of the bariatric surgery devices market. The major factor that is propelling the growth of this segment of the market is that it does not leave any wounds after the surgery, which is significantly helpful in reducing the hospital stay period.Procedure Outlook
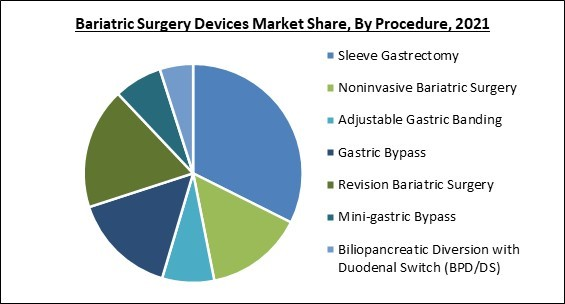
By Procedure, the Bariatric Surgery Devices Market is segregated into Sleeve Gastrectomy, Gastric Bypass, Revision Bariatric Surgery, Noninvasive Bariatric Surgery, Adjustable Gastric Banding, Mini-gastric Bypass, and Biliopancreatic Diversion with Duodenal Switch (BPD/DS). In 2021, the sleeve gastrectomy segment procured the highest revenue share of the bariatric surgery devices market. The growing benefits of sleeve gastrectomy can be ascribed to the significant proportion of this market. Compared to other bariatric operations, benefits include safety, effectiveness, price, and fewer problems.Regional Outlook
Region-Wise, the Bariatric Surgery Devices Market is analyzed across North America, Europe, Asia-Pacific, and LAMEA. In 2021, North America accounted for the largest revenue share of the bariatric surgery market. The rapidly increasing growth of the regional market is significantly attributed to factors, like the rising prevalence of chronic diseases, the incidence of obesity and diabetes, the rising number of bariatric surgeries, the rising cost of healthcare, and the rising demand for MIS surgeries.The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Johnson & Johnson, ReShape Lifesciences, Inc., Apollo Endosurgery, Inc., Medtronic PLC, Olympus Corporation, Intuitive Surgical, Inc., B. Braun Melsungen AG, Spatz FGIA, Inc., Cook Medical, Inc. (Cook Group), and Richard Wolf GmbH.
Strategies deployed in Bariatric Surgery Devices Market
- 2022-Aug: ReShape Lifesciences™ received the US FDA approval for its GIBI HD, a Gastro Intestinal Balloon Indicator. The new solution encompasses three new sizes viz. 32, 36, and 40 and aimed to streamline bariatric procedures, like laparoscopic sleeve gastrectomy, adjustable gastric banding, and gastric bypass.
- 2022-Jun: Johnson & Johnson rolled out the Echelon 3000 a next-gen stapler, in the US. The new digitally-enabled stapler aimed to offer simple and one-handed powered articulation to surgeons. Moreover, the new solution also aids in addressing the root causes of several surgical complications to enable successful navigation of the varying needs of each patient’s anatomy.
- 2022-May: ReShape Lifesciences signed an agreement with OpenLoop, an expert in full-stack virtual care delivery services. Under this agreement, the companies would offer a nationwide telehealth solution for reshapecare virtual weight loss coaching led by a professional physician.
- 2022-Jan: Johnson & Johnson collaborated with Microsoft, an American multinational technology corporation. This collaboration aimed to allow companies to expand and enable the JJMDC’s secure and compliant digital surgery ecosystem of the Johnson & Johnson Medical Devices Companies by leveraging the Microsoft Cloud's capabilities.
- 2021-Oct: Spatz FGIA received US FDA approval for its Spatz3 Gastric Balloon, the first adjustable gastric balloon system. With this approval, the company aimed to help patients with weight loss.
- 2021-Sep: Olympus launched the POWERSEAL 5mm Curved Jaw Tissue Sealer and Divider, an addition to its new POWERSEAL range of advanced bipolar surgical energy products. The new solution aimed to offer constant sealing reliability within an ergonomic and multifunctional design to comply with the highest standards of clinical performance for advanced bipolar surgical energy devices.
- 2021-Jun: ReShape Lifesciences formed a merger with Obalon Therapeutics, an engineering-driven medical technology company. Through this merger, the companies aimed to offer lucrative prospects to ReShape in order to expand its offerings of FDA-approved weight loss solutions as well as reimbursed virtual care services.
- 2021-Jun: Intuitive rolled out SureForm, a robotic-assisted surgical stapler. The new solution encompasses the ability to make automatic alterations to the firing process to help in optimizing optimize a consistent staple line and prevent damage to tissue throughout a variety of tissue thicknesses.
- 2021-Mar: Apollo Endosurgery received the Breakthrough Device Designation from the US FDA for its Orbera Intragastric Balloon. With this approval, the company aimed to aid patients in receiving access to breakthrough technologies rapidly to offer more effective diagnosis and treatment for irreversibly debilitating diseases and life-threatening conditions.
- 2021-Mar: Johnson & Johnson launched the ECHELON+ Stapler, a new powered surgical stapler with GST Reloads. The new solution aimed to enhance the staple line security while also lower complications via more uniform tissue compression as well as better staple formation.
- 2020-Jun: ReShape Lifesciences came into a partnership with inHealth Lifestyle, a Lifestyle Therapeutics company. With this partnership, the companies aimed to launch the ReShapeCare virtual health coaching program to allow physicians as well as bariatric surgeons to optimize patient management through personalized service to improve health outcomes and treatment.
- 2020-Feb: Medtronic took over Digital Surgery, a private company. Through this acquisition, the company aimed to build out its surgical robotics business of Minimally Invasive Therapies Group.
- 2018-Sep: Johnson & Johnson unveiled the Ethicon Bariatric Revision Surgical Solution, a novel and comprehensive surgical solution for bariatric revision surgery. The new solution includes a range of products along with professional expertise as well as training for managing the obstacles of reoperations and helping surgeons in performing bariatric surgeries with increased accuracy.
Scope of the Study
Market Segments Covered in the Report:
By Devices Type
- Minimally Invasive Surgical Devices
- Accessories
- Suturing Devices
- Stapling Devices
- Energy/Vessel-sealing Devices
- Noninvasive Surgical Devices
By Procedure
- Sleeve Gastrectomy
- Noninvasive Bariatric Surgery
- Adjustable Gastric Banding
- Gastric Bypass
- Revision Bariatric Surgery
- Mini-gastric Bypass
- Biliopancreatic Diversion with Duodenal Switch (BPD/DS)
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Johnson & Johnson
- ReShape Lifesciences, Inc.
- Apollo Endosurgery, Inc.
- Medtronic PLC
- Olympus Corporation
- Intuitive Surgical, Inc.
- B. Braun Melsungen AG
- Spatz FGIA, Inc.
- Cook Medical, Inc. (Cook Group)
- Richard Wolf GmbH
Unique Offerings from the Publisher
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Johnson & Johnson
- ReShape Lifesciences, Inc.
- Apollo Endosurgery, Inc.
- Medtronic PLC
- Olympus Corporation
- Intuitive Surgical, Inc.
- B. Braun Melsungen AG
- Spatz FGIA, Inc.
- Cook Medical, Inc. (Cook Group)
- Richard Wolf GmbH