Drugs are given via the skin at a predefined and controlled pace using a transdermal drug delivery system (TDDS). These methods are used to treat a variety of medical diseases, including those affecting the heart and neurological system. Skin patches can be used to deliver medications including nicotine (for stopping smoking), scopolamine (for travel sickness or motion sickness), lidocaine (for shingles pain), and nitro-glycerin (for angina).
These devices offer a number of benefits, including a longer therapeutic impact, fewer side effects, increased bioavailability, better patient compliance, and simple drug therapy termination. The appendageal, transcellular, and intercellular pathways are the three main ways that drugs can enter the body.
COVID-19 Impact Analysis
The development of the COVID-19 drug-facilitated the opening of research facilities for transdermal medication delivery systems. By the beginning of 2022, this system helped immensely in the recovery of many from the virus. With the decline in the number of infected cases, transdermal drug delivery system manufacturers have started concentrating on safeguarding their personnel, business operations, and supply networks. This is being done so they can react quickly to emergencies and implement new working procedures. Therefore, the COVID-19 pandemic had a positive impact on the transdermal drug delivery systems market.Market Growth Factors:
Increase in Self-Administration of Long-Term Drugs
Transdermal drug delivery systems have enhanced drug diffusion capabilities compared to more traditional drug administration methods, such as intravenous, oral, and pulmonary. The growing preference for pain-free medicine delivery among patients and clinicians is an important factor in the wide use of TDDS. The TDDS application is being boosted by the increased accessibility for self-administration of medications for illnesses that require long-term therapy, such as diabetic patients.Presence of Numerous Transdermal Products in Clinical Trial Phases
There are numerous active clinical trials for transdermal products that have received FDA approval currently. These trials span from Phase I through IV investigations, and the majority use transdermal devices that have previously received FDA approval for substances including fentanyl, nicotine, and hormone treatments. These studies focus on a wide range of illness states, and many are looking at new applications for already-available transdermal products or other pharmacological combinations.Marketing Restraining Factor:
Skin Layers Posing as A Barrier in Achieving Maximum Efficiency
Most transdermal drugs do not achieve their full potential because of the built-in skin barrier. The function of the skin, which has multiple layers and is the body's outermost organ, is to protect it from the outside world by shielding it from poisons, heat, and chemicals. Each layer of such skin has components that obstruct transdermal delivery, including the epidermis, which serves as a barrier, and the dermis, which houses blood vessels and generates skin cells. The permeability issue of drugs through the skin would hamper the expansion of the transdermal drug delivery systems market.Application Outlook
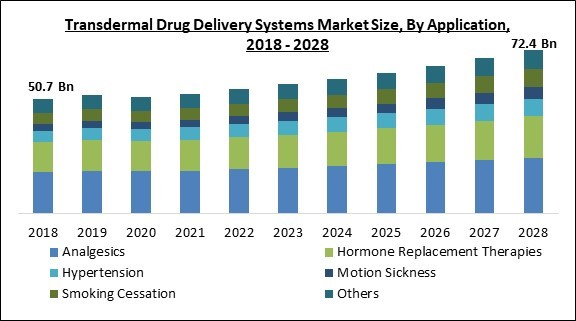
On the basis of application, the transdermal drug delivery systems market is divided into analgesics, hormone replacement therapy, hypertension, motion sickness, smoking cessation, and others. The hormone replacement therapies segment recorded a substantial revenue share in the transdermal drug delivery system market in 2021. One of their prominent use is in the menopausal symptom’s treatment.Type of Delivery System Outlook
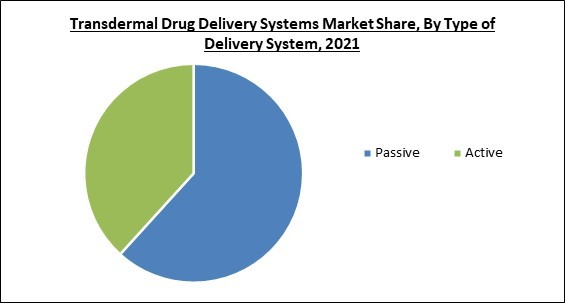
Based on type of delivery system, the transdermal drug delivery systems market is bifurcated into passive and active. The hormone replacement therapies segment recorded a substantial revenue share in the transdermal drug delivery system market in 2021. One of their prominent use is in the menopausal symptom’s treatment.Regional Outlook
Based on region, the transdermal drug delivery system is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North American region witnessed the maximum revenue share in the transdermal drug delivery systems market in 2021. Patent expirations, which lead to new businesses joining the market, are a primary driver of growth in this region. Additionally, it is believed that significant investments made by established as well as recent market entrants, the reformulation of operating drug compounds, the repositioning of previously unsuccessful drugs, and these other factors play a significant role in the sizeable share that this region has managed to capture.The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Viatris Inc., Bayer AG, Boehringer Ingelheim GmbH, Endo International plc, GlaxoSmithKline plc, Johnson & Johnson, Novartis AG, Purdue Pharma L.P., Altaris and Hisamitsu Pharmaceutical Co. Inc.
Scope of the Study
Market Segments Covered in the Report:
By Application
- Analgesics
- Hormone Replacement Therapies
- Hypertension
- Motion Sickness
- Smoking Cessation
- Others
By Type of Delivery System
- Passive
- Active
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Viatris Inc.
- Bayer AG
- Boehringer Ingelheim GmbH
- Endo International plc
- GlaxoSmithKline plc
- Johnson & Johnson
- Novartis AG
- Purdue Pharma L.P.
- Altaris
- Hisamitsu Pharmaceutical Co. Inc.
Unique Offerings from the Publisher
- Exhaustive coverage
- The highest number of market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Viatris Inc.
- Bayer AG
- Boehringer Ingelheim GmbH
- Endo International plc
- GlaxoSmithKline plc
- Johnson & Johnson
- Novartis AG
- Purdue Pharma L.P.
- Altaris
- Hisamitsu Pharmaceutical Co. Inc.