Large volume parenterals (LVPs) are sterile solutions administered intravenously in volumes exceeding 100 mL (ranging up to 2000 mL). These solutions - such as sodium chloride, dextrose, Ringer’s, and lactated Ringer’s - are critical for fluid replacement, electrolyte balance, nutrition, and rapid medication delivery, particularly in surgical and chronic care settings.
Market Drivers
- Chronic Disease Burden: Rising global prevalence of conditions requiring hospitalization (e.g., cancer, diabetes, renal disorders) fuels demand for LVPs in fluid and nutrient therapy.
- Surgical Volume Growth: Increasing surgical procedures, where LVPs are essential for anesthesia, hydration, and post-operative care, drive market expansion.
- Advancements in Drug Delivery: LVPs enable efficient, continuous administration of medications like morphine for cancer pain management, aligning with the demand for single-dose therapies and streamlined treatment protocols.
Geographical Insights
- North America leads the market, supported by robust healthcare infrastructure and high rates of chronic pain conditions. For example, the American Cancer Society projects 2,001,140 new cancer cases and 611,720 deaths in the U.S. in 2024, underscoring the need for LVPs in pain management.
- The region’s growing surgical volumes and emphasis on advanced fluid replacement therapies further solidify its dominance.
Reasons for buying this report:
- Insightful Analysis: Gain detailed market insights covering major as well as emerging geographical regions, focusing on customer segments, government policies and socio-economic factors, consumer preferences, industry verticals, other sub-segments.
- Competitive Landscape: Understand the strategic maneuvers employed by key players globally to understand possible market penetration with the correct strategy.
- Market Drivers & Future Trends: Explore the dynamic factors and pivotal market trends and how they will shape up future market developments.
- Actionable Recommendations: Utilize the insights to exercise strategic decision to uncover new business streams and revenues in a dynamic environment.
- Caters to a Wide Audience: Beneficial and cost-effective for startups, research institutions, consultants, SMEs, and large enterprises.
What do businesses use our reports for?
Industry and Market Insights, Opportunity Assessment, Product Demand Forecasting, Market Entry Strategy, Geographical Expansion, Capital Investment Decisions, Regulatory Framework & Implications, New Product Development, Competitive Intelligence.Report Coverage:
- Historical data & forecasts from 2022 to 2030
- Growth Opportunities, Challenges, Supply Chain Outlook, Regulatory Framework, Customer Behaviour, and Trend Analysis
- Competitive Positioning, Strategies, and Market Share Analysis
- Revenue Growth and Forecast Assessment of segments and regions including countries
- Company Profiling (Strategies, Products, Financial Information, and Key Developments among others)
The large volume parenteral market is segmented and analyzed as follows:
- By Volume
- 100 ml - 250 ml
- 250 ml - 500 ml
- Above 500 ml
- By Application
- Correction of electrolyte & fluid balance disturbances
- Nutrition
- Vehicle for administering other drugs
- By Packaging Container
- Glass Bottle
- Plastic Bags
- By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- UK
- Germany
- France
- Italy
- Others
- Middle East and Africa
- UAE
- Israel
- Saudi Arabia
- Others
- Asia Pacific
- Japan
- China
- India
- South Korea
- Taiwan
- Thailand
- Indonesia
- Others
- North America
Table of Contents
Companies Mentioned
- Fresenius Kabi AG
- Albert David Ltd.
- Otsuka Holdings Co., Ltd.
- Grifols S.A.
- B. Braun Melsungen AG
- BML Parenteral Drugs
- Akums Drugs & Pharmaceuticals Ltd.
- DJ Labs
- Parenteral Drugs (India) Limited
- BKRS Pharma Pvt. Ltd.
- Pfizer.
- Baxter.
- August Bioservices
- INCOG BioPharma Services
- Simtra BioPharma Solutions
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 137 |
| Published | December 2024 |
| Forecast Period | 2025 - 2030 |
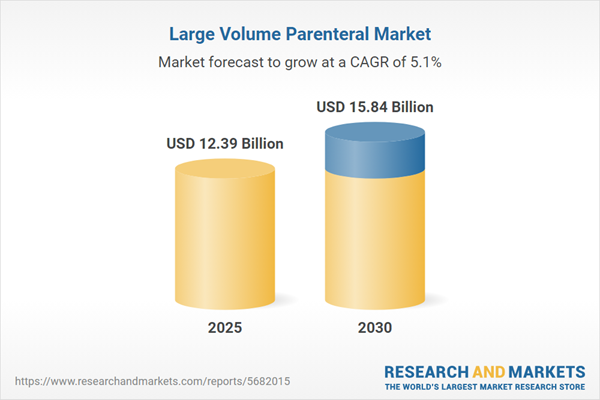
| Estimated Market Value ( USD | $ 12.39 Billion |
| Forecasted Market Value ( USD | $ 15.84 Billion |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |