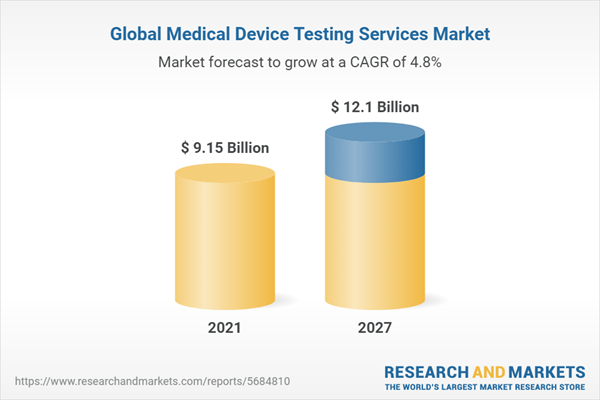
The global medical device testing services market was valued at USD 9.15 billion in 2021, growing at a CAGR of 4.82% during the forecast period from 2022 to 2027 to reach USD 12.10 billion by 2027. The medical device testing services market is witnessing positive growth owing to various factors such as the growing necessity for validation and verification of medical devices due to strict government regulations. Furthermore, the ongoing technological development in the medical industry and the rise in small medical device firms without in-house expertise across the globe will propel the demand of medical device testing services. Additionally, the integration of smartphones, artificial intelligence, Iot services with the medical devices are offering prominent opportunities for market growth. Therefore, the market for medical device testing services are estimated to grow at a significant CAGR during the forecast period from 2022 to 2027.
MEDICAL DEVICE TESTING SERVICES MARKET DYNAMICS:
One of the main drivers of the medical device testing services market is the increasing overall medical device industry owing to the rise in general patient pool suffering from the various chronic diseases. Moreover, the rising investments by small medical devices firms along with key players will upsurge the demand of medical device testing services in the given forecast period.According to the World Health Organization (WHO) 2022, chronic diseases kills nearly 41 million people each year, equivalent to 74% of all deaths globally. The main types of chronic diseases are cardiovascular diseases such as heart attacks and stroke, cancers, chronic respiratory diseases such as chronic obstructive pulmonary disease and asthma, diabetes, and others. Also, the source listed the incidence of cancer in 2020, globally. The most common form of cancers were reported was of breast cancer with 2.26 million cases, lung cancer -2.21 million cases, colon and rectum cancer with 1.93 million cases, prostate cancer with 1.41 million cases, skin cancer 1.20 million cases, and stomach cancer with 1.09 million cases, globally.
The increasing incidence of cancers will increase the demand of medical devices in the market used for diagnosis, treatment or management of the disease, ultimately increasing the demand of medical device testing services.
Further, the strict regulations by the government institutes or organizations for validation and verification of medical devices will surge the demand of medical device testing service. Also, the incorporation of artificial intelligence (AI) and IoT services in the device will increase the demand of medical device testing services as the AI devices are always at a risk of cyber risks, and privacy issues so it is very essential to validate and verify all the risks associated with these medical device.
Therefore, the factors stated above collectively will drive the overall medical device testing services market during the forecast period from 2022-2027.
However, high cost of medical devices, presence of different regulatory authorties in different regions, and others, can hamper the global medical device testing services market growth.
The COVID-19 epidemic has positively impacted the medical device testing services market due to the increasing demand for COVID-19-related products such as masks, PPE, respiratory equipment, and others. The demand of the ventilators, COVID-19 vaccines, IVD kits, and other products increases as they were used to either manage or treat or prevent the infection. The medical device testing services is required for the above mentioned devices or products. Thus, the increasing demand of the various medical devices during the pandemic increased the demand of the medical device testing services, ultimately propelling the overall market. Additionally, with the increasing demand of the medical device many key players increased their medical device manufacturing, thereby increasing the demand of testing services. Moreover, many key players working in the market of medical device testing services provided remote inspections of medical equipment to maintain the continuity of health and safety compliance and avoid any possible physical contact. Henceforth, the above factors increased the demand of the medical device testing services and is likely to do the same in the upcoming years.
MEDICAL DEVICE TESTING SERVICES MARKET SEGMENT ANALYSIS:
Medical Device Testing Services Market By Service Type (Testing Service, Inspection Service, and Certification Service), Sourcing Type (In-House and Outsourced), Device Class (Class I, Class II, and Class III), Product Type (In-Vitro Diagnostic Medical Device, Active Implant Medical Device, Vascular Medical Device, Ophthalmic Medical Device, and Others), and Geography (North America, Europe, Asia-Pacific, and Rest of the World)In the service type segment of the medical device testing services, the testing service market is expected to have a significant revenue share in the year 2021. This was primarily owing to the high demand for quality and safe products and due to the strong recommendations from regulatory authorities to ensure the safety, quality, and efficiency of the products.
For instance, examples of testing services are electro medical device testing, biocompatibility testing, clinical research services which help in assessing the final product to investigate the faulty material, and testing the performance of medical devices and the electrical safety of devices.
Further, the testing services are carried out in laboratories and research sites. Also, the testing services assist manufacturers in improving the marketability of their medical devices and in lowering costs in the pre-production phase e.g. R&D, the selection of suppliers, and others.
Moreover, key players in the market are expanding their testing services portfolio. For instance, in June 2020, Intertek, announced the expansion of its personal protective equipment services to include the pre-certification testing of N95 respirators to requirements set by the National Institute for Occupational Safety and Health (NIOSH).
Thus, due to the interplay of all the factors mentioned above, this can result in the rising demand for testing services, which in turn would provide a conducive growth environment for overall medical device testing services market during the market forecast 2022-2027.
NORTH AMERICA IS EXPECTED TO DOMINATE THE OVERALL MEDICAL DEVICE TESTING SERVICES MARKET:
Among all the regions, North America is expected to dominate the global medical device testing services market in the year 2021 and is expected to do the same during the forecast period from 2022-2027. Factors such as strict regulations established by the governments to maintain quality and safety standards in the industry, rising per capita income among the region, growing consumer awareness regarding the importance of certification, and others will increase the demand for medical device testing services in the North America market. Further, the new and replacement sales, rising country demographics, surging disease epidemiology, and import-export tariffs of the medical devices in the region will increase the demand of medical device testing services in the given forecast period.Also, the increasing prevalence of chronic disorders such as cardiovascular, respiratory, and other disorders will increase the demand of medical devices in the region, ultimately surging the need of medical device testing services. For instance, according to the Centers for Disease Control and Prevention (CDC) 2022, about 20.1 million adults age 20 and older had coronary artery disease in 2020 in the United States. Also, in the United States, every year, about 805,000 people in the United States have a heart attack. Thus, the increasing prevalence of cardiovascular disorders will increase the demand of the medical devices used in diagnosis or treatment or management of the disease.
Further, the increasing complexity in product design and growing efforts toward cost-cutting is one of the factor responsible for driving the market growth. Besides, the presence of stringent regulatory bodies such as the FDA is fueling the market growth in the region. The rapid increase in the manufacturing of medical devices to meet the high demand for efficient healthcare in the region is expected to be one of the major factors that can be attributed to the market growth in North America.
Therefore, the interplay of the aforementioned factors above would provide a conducive growth environment for the North American region in the medical device testing services.
MEDICAL DEVICE TESTING SERVICES MARKET KEY PLAYERS:
Some of the key market players operating in the medical device testing services market include Element Minnetonka, Bioneeds, Charles River Laboratories., A Sotera Health company, North American Science Associates, LLC, Labcorp, SGS Société Générale de Surveillance SA, Eurofins Scientific, Pace Analytical Services LLC, Intertek Group plc, Bureau Veritas, TÜV SÜD, DEKRA, Element Materials Technology, Medistri SA, UL LLC, The British Standards Institution, Biomedical Device Labs, NTS, ImpactQA., and others.RECENT DEVELOPMENTAL ACTIVITIES IN THE MEDICAL DEVICE TESTING SERVICES MARKET:
- In April 2021, TÜV SÜD announced that it had presented itself at Medtec LIVE to exhibit its ability to be a one-stop-shop for medical device testing. The company's services covered testing in the areas of electrical and functional safety, cyber security and software, EMC, and biocompatibility. The experts from TÜV SÜD featured in the online trade show and congress program with various talks, a live hack and an elevator pitch.
- In October 2019, TÜV SÜD signed a memorandum of understanding (MoU) with the NUS Centre for Additive Manufacturing (AM.NUS) at the National University of Singapore (NUS), to promote R&D activities in the area of 3D printed biomedical implants for clinical trials.
- In May 2019, Intertek announced the expansion of its pharmaceutical services laboratory in Melbourne, near Cambridge, through the acquisition of a new 20,000 sq. ft. facility, which will double the footprint of the existing laboratory.
- In February 2019, Bureau Veritas and Microsoft, the leader in productivity platforms and services, announced the conclusion of a global technical and business collaboration for the development of laboratory testing services based on artificial intelligence (AI).
KEY TAKEAWAYS FROM THE MEDICAL DEVICE TESTING SERVICES MARKET REPORT STUDY
- Market size analysis for current medical device testing services (2021), and market forecast for 5 years (2022-2027)
- The effect of the COVID-19 pandemic on this market is significant. To capture and analyze suitable indicators, our experts are closely watching the medical device testing services market
- Top key product/services/technology developments, mergers, acquisitions, partnerships, and joint ventures happened over the last 3 years
- Key companies dominating the global medical device testing services market
- Various opportunities are available for the other competitor in the medical device testing services market space.
- What are the top-performing segments in 2021? How these segments will perform in 2027.
- Which are the top-performing regions and countries in the current medical device testing services market scenario?
- Which are the regions and countries where companies should have concentrated on opportunities for medical device testing services market growth in the coming future?
TARGET AUDIENCE WHO CAN BE BENEFITED FROM THIS MEDICAL DEVICE TESTING SERVICES MARKET REPORT STUDY
- Medical device testing services providers
- Research organizations and consulting companies
- Medical device testing services market-related organizations, associations, forums, and other alliances
- Government and corporate offices
- Start-up companies, venture capitalists, and private equity firms
- Distributors and traders dealing in medical device testing services market
- Various end-users who want to know more about the medical device testing services and the latest technological developments in the medical device testing services market
FREQUENTLY ASKED QUESTIONS FOR MEDICAL DEVICE TESTING SERVICES MARKET:
1. What are medical device testing services?
Medical device testing is the process of demonstrating that the device will reliably and safely perform in use. In new product development, extensive Design Validation Testing is applied. This includes performance testing, toxicity and chemical analysis, and sometimes human factors or even clinical testing. Ongoing quality assurance testing is generally more limited. This will usually include dimensional checks, some functional tests, and packaging verification2.What is the global market for medical device testing services?
The global medical device testing services market was valued at USD 9.15 billion in 2021, growing at a CAGR of 4.82% during the forecast period from 2022 to 2027 to reach USD 12.10 billion by 2027.3. What are the drivers for the global medical device testing services market?
The medical device testing services is witnessing an affirmative market growth due to the factors such as the increasing need for validation and verification of medical devices, ongoing technological development in the medical industry, rise in small medical device firms without in-house expertise, and others.4. Who are the key players operating in the medical device testing services market?
Some of the key market players operating in the medical device testing services include Element Minnetonka, Bioneeds, Charles River Laboratories., A Sotera Health company, North American Science Associates, LLC, Labcorp, SGS Société Générale de Surveillance SA, Eurofins Scientific, Pace Analytical Services LLC, Intertek Group plc, Bureau Veritas, TÜV SÜD, DEKRA, Element Materials Technology, Medistri SA, UL LLC, The British Standards Institution, Biomedical Device Labs, NTS, ImpactQA., and others5. Which region has the highest share in medical device testing services market?
North America is expected to dominate the global medical device testing services. Factors contributing to the growth are strict regulations established by the governments to maintain quality and safety standards in the industry, rising per capita income among the region, growing consumer awareness regarding the importance of certification, and others, which are contributing to the growth of the medical device testing services in the North America region.This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Element Minnetonka
- Bioneeds
- Charles River Laboratories.
- A Sotera Health company
- North American Science Associates, LLC
- Labcorp
- SGS Société Générale de Surveillance SA
- Eurofins Scientific
- Pace Analytical Services LLC
- Intertek Group plc
- Bureau Veritas
- TÜV SÜD
- DEKRA
- Element Materials Technology
- Medistri SA
- UL LLC
- The British Standards Institution
- Biomedical Device Labs
- NTS
- ImpactQA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 100 |
| Published | July 2024 |
| Forecast Period | 2021 - 2027 |
| Estimated Market Value ( $ | $ 9.15 Billion |
| Forecasted Market Value ( $ | $ 12.1 Billion |
| Compound Annual Growth Rate | 4.8% |
| Regions Covered | Global |