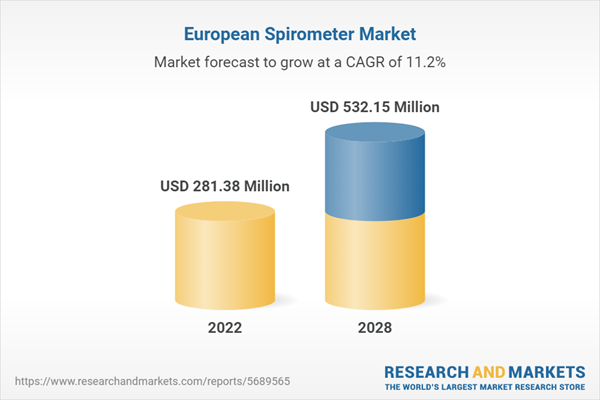
The spirometer market in Europe is expected to grow from US$ 281.38 million in 2022 to US$ 532.15 million by 2028. It is estimated to grow at a CAGR of 11.2% from 2022 to 2028.
Transplantation outcomes has been improved with advances in operative techniques. However, optimal management of infection and organ rejection remains problematic because the complications may be undetectable in the early phases of routine clinical evaluation. In such cases, home spirometry is a useful instrument that can measure lung function, help monitor the progress post-lung transplantation, and aid in the early detection of allograft dysfunction.
Home spirometry offers advantages over clinic spirometry as it provides increased patient convenience and more frequent lung function measurements, enabling early disease progression detection. Moreover, home spirometers are widely used to provide easy access to more detailed information regarding lung function. Spirometry is also used before lung cancer surgery to predict how well a patient will tolerate the operation and manage it once a portion or lobe of a lung is removed.
In addition, one such spirometer is alveoair, a personal spirometer designed for asthma, COPD, and cystic fibrosis patients to help quantify their lung health. It enables patients to monitor their disease at home to help avoid attacks and unnecessary hospital visits. Alveoair is a clinically trialed spirometer that has completed its clinical trial in January 2021 and provides results at par with a hospital-grade spirometer. Furthermore, home monitoring of physiological variables has become progressively more practical with the miniaturization and falling costs of devices and consequent improvements in wireless electronic connectivity. Additionally, within respiratory medicine, home disease monitoring in the form of peak flow measurement is already a feature of asthma self-management. Daily surveillance spirometry is used to detect acute rejection episodes in lung transplantation recipients and has been preferred by many patients. By using home spirometry and daily check of its result, healthcare givers can closely observe the patients and help them manage their health at home. Thus, the increasing preference for home care products is driving the spirometer market growth.
A few key players dominating the Europe spirometer market are Baxter International Inc.; COSMED Srl; Depisteo LLC.; Henry Schein, Inc.; ICU Medical Inc.; NUVOAIR US, INC. (NUVOAIR AB); Teleflex Incorporated; and VYAIRE MEDICAL, INC.
Transplantation outcomes has been improved with advances in operative techniques. However, optimal management of infection and organ rejection remains problematic because the complications may be undetectable in the early phases of routine clinical evaluation. In such cases, home spirometry is a useful instrument that can measure lung function, help monitor the progress post-lung transplantation, and aid in the early detection of allograft dysfunction.
Home spirometry offers advantages over clinic spirometry as it provides increased patient convenience and more frequent lung function measurements, enabling early disease progression detection. Moreover, home spirometers are widely used to provide easy access to more detailed information regarding lung function. Spirometry is also used before lung cancer surgery to predict how well a patient will tolerate the operation and manage it once a portion or lobe of a lung is removed.
In addition, one such spirometer is alveoair, a personal spirometer designed for asthma, COPD, and cystic fibrosis patients to help quantify their lung health. It enables patients to monitor their disease at home to help avoid attacks and unnecessary hospital visits. Alveoair is a clinically trialed spirometer that has completed its clinical trial in January 2021 and provides results at par with a hospital-grade spirometer. Furthermore, home monitoring of physiological variables has become progressively more practical with the miniaturization and falling costs of devices and consequent improvements in wireless electronic connectivity. Additionally, within respiratory medicine, home disease monitoring in the form of peak flow measurement is already a feature of asthma self-management. Daily surveillance spirometry is used to detect acute rejection episodes in lung transplantation recipients and has been preferred by many patients. By using home spirometry and daily check of its result, healthcare givers can closely observe the patients and help them manage their health at home. Thus, the increasing preference for home care products is driving the spirometer market growth.
Europe Spirometer Market Segmentation
The Europe spirometer market is segmented by type, technology, application, and country. Based on type, the market is segmented into handheld spirometers, table-top spirometers, and desktop spirometers. The table-top spirometers segment dominated the market in 2022. By technology, the market is segmented into volume, flow, and peak flow. The flow segment dominated the market in 2022. By application, the market is segmented into asthma, chronic obstructive pulmonary disease, cystic fibrosis, and others. The chronic obstructive pulmonary disease segment dominated the market in 2022. Based on country, the market is segmented into Germany, the UK, France, Italy, Spain, and the rest of Europe. Further, Germany dominated the market in 2022.A few key players dominating the Europe spirometer market are Baxter International Inc.; COSMED Srl; Depisteo LLC.; Henry Schein, Inc.; ICU Medical Inc.; NUVOAIR US, INC. (NUVOAIR AB); Teleflex Incorporated; and VYAIRE MEDICAL, INC.
Table of Contents
1. Introduction
3. Research Methodology
4. Europe Spirometer Market - Market Landscape
5. Europe Spirometer Market - Key Market Dynamics
6. Spirometer Market- Europe Analysis
7. Europe Spirometer Market- by Type
8. Europe Spirometer Market - by Technology
9. Europe Spirometer Market- by Application
10. Europe Spirometer Market- Country Analysis
11. Europe Spirometers Market-Industry Landscape
12. Company Profiles
13. Appendix
List of Tables
List of Figures
Companies Mentioned
- Baxter International Inc.
- COSMED Srl
- Depisteo LLC.
- Henry Schein, Inc.
- ICU Medical Inc.
- NUVOAIR U.S, INC. (NUVOAIR AB)
- Teleflex Incorporated
- VYAIRE MEDICAL, INC.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 126 |
| Published | October 2022 |
| Forecast Period | 2022 - 2028 |
| Estimated Market Value ( USD | $ 281.38 Million |
| Forecasted Market Value ( USD | $ 532.15 Million |
| Compound Annual Growth Rate | 11.2% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 8 |