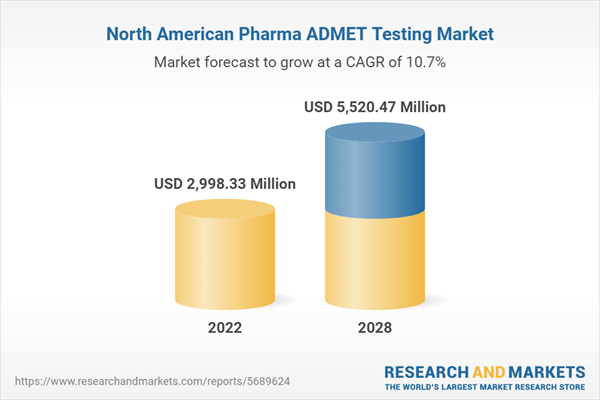
The pharma ADMET testing market in North America is expected to grow from US$ 2,998.33 million in 2022 to US$ 5,520.47 million by 2028. It is estimated to grow at a CAGR of 10.7% from 2022 to 2028.
The drug approval process is projected to take 15 years, and phases II and III are the most expensive phases of clinical trials. According to the study titled`Why 90% of Clinical Drug Development Fails and How to Improve It?`, 2022, 90% of clinical drug development fails despite implementing many strategies. After entering clinical investigations, 9 out of 10 drug candidates fail during phase I, II, and III clinical trials and the drug approval process. Drug candidates rejected in preclinical stages are not included in the 90% failure rate of the drugs in clinical stages, as they do not enter the phase I clinical trials. If preclinical drug candidates are included, the drug discovery and development failure rate rise even higher than 90%. According to analyses of clinical trial data from 2010 to 2017, lacking clinical effectiveness (40-50%), uncontrollable toxicity (30%), poor drug-like qualities (10-15%), no commercial needs, and ineffective strategic planning (10%) are the 4 major causes of trial failure.
New drug development is both resource and time-intensive, where later clinical stages result in high costs. To lower the attrition rate in drug research & development, it is crucial to filter and optimize pharmaceuticals' absorption, distribution, metabolism, elimination, and toxicity (ADMET) characteristics at an early stage. It has been widely accepted that drug ADMET properties should be considered as early as possible to reduce failure rates in the clinical phase of drug discovery. In vitro and in vivo drug evaluation techniques have reached maturity in preclinical applications, and in silico technologies are gaining vast acceptance to evaluate the relevant properties of drugs in the preclinical stage. The development of software programs and in silico models is further promoting the implementation of ADMET studies. Thus, the increasing need for drug-development ADME testing boosts the pharma ADMET testing market .
A few key players dominating the North America pharma ADMET testing market are Agilent Technologies, Inc.; Bio-Rad Laboratories, Inc.; Biovia (Dassault Systèmes); Charles River Laboratories; CMIC HOLDINGS Co., LTD.; Cyprotex Limited; IQVIA Inc.; MERCK KGaA; Promega Corporation; and Wuxi AppTec.
The drug approval process is projected to take 15 years, and phases II and III are the most expensive phases of clinical trials. According to the study titled`Why 90% of Clinical Drug Development Fails and How to Improve It?`, 2022, 90% of clinical drug development fails despite implementing many strategies. After entering clinical investigations, 9 out of 10 drug candidates fail during phase I, II, and III clinical trials and the drug approval process. Drug candidates rejected in preclinical stages are not included in the 90% failure rate of the drugs in clinical stages, as they do not enter the phase I clinical trials. If preclinical drug candidates are included, the drug discovery and development failure rate rise even higher than 90%. According to analyses of clinical trial data from 2010 to 2017, lacking clinical effectiveness (40-50%), uncontrollable toxicity (30%), poor drug-like qualities (10-15%), no commercial needs, and ineffective strategic planning (10%) are the 4 major causes of trial failure.
New drug development is both resource and time-intensive, where later clinical stages result in high costs. To lower the attrition rate in drug research & development, it is crucial to filter and optimize pharmaceuticals' absorption, distribution, metabolism, elimination, and toxicity (ADMET) characteristics at an early stage. It has been widely accepted that drug ADMET properties should be considered as early as possible to reduce failure rates in the clinical phase of drug discovery. In vitro and in vivo drug evaluation techniques have reached maturity in preclinical applications, and in silico technologies are gaining vast acceptance to evaluate the relevant properties of drugs in the preclinical stage. The development of software programs and in silico models is further promoting the implementation of ADMET studies. Thus, the increasing need for drug-development ADME testing boosts the pharma ADMET testing market .
North America Pharma ADMET Testing Market Segmentation
The North America pharma ADMET testing market is segmented by testing type, technology, application, and country. Based on testing type, the market is segmented into in vivo ADMET testing, in vitro ADMET testing, and in silico ADMET testing. The in vivo ADMET testing segment is dominating the market in 2022. Based on technology, the market is segmented into cell culture, high throughput, molecular imaging, and OMICS technology. The cell culture segment is dominating the market in 2022. Based on application, the market is segmented into systemic toxicity, renal toxicity, hepatotoxicity, neurotoxicity, and others. The systemic toxicity segment is dominating the market in 2022. Based on country, the market is segmented into the US, Canada, and Mexico. Further, the US is dominating the market in 2022.A few key players dominating the North America pharma ADMET testing market are Agilent Technologies, Inc.; Bio-Rad Laboratories, Inc.; Biovia (Dassault Systèmes); Charles River Laboratories; CMIC HOLDINGS Co., LTD.; Cyprotex Limited; IQVIA Inc.; MERCK KGaA; Promega Corporation; and Wuxi AppTec.
Table of Contents
1. Introduction
3. Research Methodology
4. North America Pharma ADMET Testing Market - Market Landscape
5. Pharma ADMET Testing Market - Key Market Dynamics
6. Pharma ADMET Testing Market- North America Analysis
7. North America Pharma ADMET Testing Market- By Testing Type
8. North America Pharma ADMET Testing Market- By Technology
9. North America Pharma ADMET Testing Market- By Application
10. North America Pharma ADMET Testing Market- Country Analysis
11. North America Pharma ADMET Testing Market-Industry Landscape
12. Company Profiles
13. Appendix
List of Tables
List of Figures
Companies Mentioned
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Biovia (Dassault Systèmes)
- Charles River Laboratories
- CMIC HOLDINGS Co., LTD
- Cyprotex Limited
- IQVIA Inc.
- MERCK KGaA
- Promega Corporation
- Wuxi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 131 |
| Published | October 2022 |
| Forecast Period | 2022 - 2028 |
| Estimated Market Value ( USD | $ 2998.33 Million |
| Forecasted Market Value ( USD | $ 5520.47 Million |
| Compound Annual Growth Rate | 10.7% |
| Regions Covered | North America |
| No. of Companies Mentioned | 10 |