Embolic protection devices are specialized medical tools designed to prevent the migration of debris, blood clots, or other potentially harmful particles during minimally invasive procedures, particularly in cardiovascular interventions. These devices act as filters or barriers, capturing and containing emboli that could otherwise travel through the bloodstream and cause complications such as stroke or organ damage. By safeguarding vital arteries and reducing the risk of embolization, these devices improve the safety and success rates of procedures like angioplasty, stent placement, and atherectomy. As a result, embolic protection devices find extensive applications across the healthcare industry as they play a crucial role in preserving patient well-being by minimizing the potential for post-procedural complications arising from embolism.
The rising demand for embolic protection devices to mitigate the risks of embolization during interventions due to the increasing prevalence of cardiovascular diseases such as atherosclerosis and coronary artery disease will stimulate the growth of the embolic protection devices market during the forecast period. Moreover, the increasing adoption of minimally invasive techniques across various medical specialties has augmented the demand for embolic protection devices. These devices are integral to preserving patient safety during complex procedures like angioplasty, stenting, and transcatheter aortic valve replacement. Apart from this, numerous technological advancements, including improved filter designs and material innovation, that enhance performance and patient outcomes, are propelling the market growth. Furthermore, the heightened emphasis on reducing post-procedural complications and hospital stays has accelerated the adoption of embolic protection devices as it assists in preventing embolic events, thereby contributing to the market growth.
Embolic Protection Devices Market Trends/Drivers:
Rise in prevalence of cardiovascular diseases
The surge in cardiovascular diseases like atherosclerosis and coronary artery diseases acts as a primary driver in the embolic protection device market. With an aging global population and lifestyle factors contributing to health concerns worldwide, interventions such as angioplasty and stent placement are becoming more prevalent. Embolic protection devices play a critical role in mitigating the risks of embolization, a potential complication that can lead to severe consequences like stroke. By capturing and containing debris during these procedures, these devices significantly improve patient safety and overall procedural outcomes. The growing demand for embolic protection devices stems from healthcare professionals' commitment to addressing the mounting burden of cardiovascular diseases globally and minimizing the associated procedural risks.Growing inclination toward minimally invasive procedures
The increasing adoption of minimally invasive techniques across diverse medical specialties stimulates the demand for embolic protection devices. These devices are essential for ensuring patient safety during procedures like transcatheter interventions, where the risk of embolization is significant. As patients and healthcare providers gravitate toward less invasive approaches, offering quicker recovery and shorter hospital stays, the need for effective embolic protection becomes paramount. The adaptability of these devices to various minimally invasive (MI) procedures underscores their significance in modern medical practice, where they play a crucial role in optimizing patient outcomes and procedural success.Rapid technological advancements
Continuous innovations in device technology, including refined filter designs, advanced materials, and improved deployment mechanisms, are pivotal drivers of the embolic protection device market. Healthcare professionals seek tools that deliver enhanced performance and safety during interventions. The evolution of embolic protection devices effectively addresses these demands, providing better debris capture, streamlined procedures, and improved patient outcomes. As the medical field increasingly prioritizes novel solutions that enhance both procedural efficacy and patient well-being, the quest for sophisticated embolic protection devices propel the market forward, offering significant benefits to medical practitioners and the patients they serve.Embolic Protection Devices Industry Segmentation:
The publisher provides an analysis of the key trends in each segment of the global embolic protection devices market report, along with forecasts at the global, regional and country levels from 2025-2033. Our report has categorized the market based on product, application, procedure, material, usage and end-user.Breakup by Product:
- Distal Filter Devices
- Distal Occlusion Devices
- Proximal Occlusion Devices
Distal filter devices dominate the market
The report has provided a detailed breakup and analysis of the market based on the product. This includes distal filter, distal occlusion, and proximal occlusion devices. According to the report, distal filter devices represented the largest segment.Distal filter devices have emerged as a driving force within the embolic protection devices market due to their pivotal role in minimizing embolic events during interventional procedures. These devices employ finely engineered filters positioned downstream to capture and contain debris released during procedures such as transcatheter interventions and angioplasty. By effectively intercepting emboli and preventing their migration to vital vessels, distal filter devices significantly mitigate the risk of complications, notably strokes.
Moreover, the growing recognition of their efficacy in preserving cerebral and peripheral circulation has spurred their adoption among healthcare professionals, leading to an increased demand for such devices. As a result, distal filter devices have enhanced patient outcomes and safety while supporting the overall growth trajectory of the embolic protection devices industry, underscoring their significance in modern medical interventions.
Breakup by Application:
- Coronary Artery Treatment
- Carotid Artery Treatment
- Others
Coronary artery treatment holds the largest share in the market
A detailed breakup and analysis of the market based on the application has also been provided in the report. This includes coronary artery treatment, carotid artery treatment, and others. According to the report, coronary artery treatment accounted for the largest market share.Coronary artery treatment devices exert a substantial influence on the embolic protection devices market by addressing the inherent risk of embolic events in coronary interventions. These devices encompass a wide range of advanced technologies, including stents, angioplasty balloons, and atherectomy systems, all of which hold the potential to dislodge embolic debris during procedures. To counter this risk, embolic protection devices are integrated to capture and contain such debris, preventing its migration and minimizing adverse outcomes.
Furthermore, the growing demand for effective embolic protection strategies in coronary interventions has accelerated the adoption of dedicated embolic protection devices, as they strengthen the safety profile of coronary procedures, reducing the likelihood of complications such as myocardial infarctions and strokes. This symbiotic relationship between coronary artery treatment devices and embolic protection devices underscores their collective role in enhancing patient care and shaping the evolving landscape of cardiovascular interventions.
Breakup by Procedure:
- Percutaneous Coronary Intervention
- Carotid Artery Stenosis
- Saphenous Vein Graft Intervention
- Aortic Valve Stenosis (Transcatheter Aortic Valve Replacement)
- Others
Percutaneous coronary intervention represents the most widely used procedure
The report has provided a detailed breakup and analysis of the market based on the procedure. This includes percutaneous coronary intervention, carotid artery stenosis, saphenous vein graft intervention, aortic valve stenosis (transcatheter aortic valve replacement), and others. According to the report, percutaneous coronary intervention represented the largest segment.Percutaneous coronary intervention (PCI) significantly influences the embolic protection devices market due to its widespread use in treating coronary artery disease. PCI procedures involve the deployment of stents and other devices to open narrowed or blocked coronary arteries. However, these interventions carry the risk of embolic debris dislodgement, potentially leading to adverse events.
Furthermore, embolic protection devices play a crucial role in mitigating this risk by capturing and containing such debris, thereby reducing the chance of downstream embolization and associated complications. As PCI procedures continue to be a cornerstone of coronary artery disease management, the demand for effective embolic protection strategies has increased, fueling the growth of the market for embolic protection devices. This interdependence underscores the vital role of embolic protection devices in fortifying the safety and efficacy of percutaneous coronary interventions.
Breakup by Material:
- Nitinol
- Polyurethane
Nitinol represents the most popular material
A detailed breakup and analysis of the market based on the material has also been provided in the report. This includes nitinol and polyurethane. According to the report, nitinol accounted for the largest market share.Nitinol, a shape memory alloy with remarkable flexibility and thermal properties, plays a crucial role in driving the embolic protection devices market. This material's unique attributes make it an ideal choice for constructing filters and frames in embolic protection devices. Nitinol's ability to undergo controlled deformation and return to its original shape when exposed to heat enables the creation of intricate yet resilient designs that can navigate tortuous vessels while maintaining structural integrity. This exceptional combination of properties enhances the performance of embolic protection devices by ensuring optimal vessel coverage and debris capture. As healthcare professionals seek devices that offer both efficacy and adaptability during interventions, Nitinol-based embolic protection devices stand out as an essential driver in shaping the market's growth, ultimately contributing to improved patient outcomes in various vascular procedures.
Breakup by Usage:
- Disposable Devices
- Re-Usable Devices
Disposable devices account for the majority of the market share
A detailed breakup and analysis of the market based on the usage has also been provided in the report. This includes disposable and re-usable devices. According to the report, disposable devices accounted for the largest market share.Disposable devices aids in addressing critical concerns related to infection control, convenience, and cost-effectiveness. These disposable devices, designed for single-use applications, eliminate the need for reprocessing and sterilization, thereby minimizing the risk of cross-contamination and enhancing procedural efficiency. Moreover, they offer healthcare providers a hassle-free solution that reduces the burden of equipment maintenance.
Furthermore, the escalating demand for infection prevention measures and streamlined workflows has fueled the adoption of disposable embolic protection devices in various interventional procedures, spanning from endovascular interventions to transcatheter techniques. As medical settings prioritize patient safety and operational ease, the prominence of disposable embolic protection devices continues to escalate, substantiating their pivotal role in catalyzing the growth and evolution of the embolic protection devices market.
Breakup by End-User:
- Hospitals and Clinics
- Ambulatory Surgical Centers
- Others
Hospitals and clinics represent the leading end user segment
A detailed breakup and analysis of the market based on the end user has also been provided in the report. This includes hospitals and clinics, ambulatory surgical centers, and others. According to the report, hospitals and clinics accounted for the largest market share.As healthcare facilities, such as hospitals and clinics, continually strive to enhance patient outcomes and safety, the demand for effective embolic protection strategies has grown.
Moreover, hospitals and clinics actively seek advanced embolic protection devices to integrate into various interventional procedures, such as angioplasty, stent placement, and transcatheter interventions. The imperative to minimize the risk of embolic events, coupled with the need for streamlined procedural workflows, accelerates the adoption of these devices. Furthermore, as healthcare providers prioritize evidence-based practices, the utilization of embolic protection devices aligns with best practices for reducing complications and improving patient care, thus solidifying hospitals and clinics as key drivers in shaping the embolic protection devices market's expansion.
Breakup by Region:
- North America
- United States
- Canada
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Others
- Latin America
- Brazil
- Mexico
- Others
- Middle East and Africa
North America exhibits a clear dominance in the market
The report has also provided a comprehensive analysis of all the major regional markets, which include North America (the United States and Canada); Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, and others); Europe (Germany, France, the United Kingdom, Italy, Spain, Russia, and others); Latin America (Brazil, Mexico, and others); and the Middle East and Africa. According to the report, North America accounted for the largest market share.North America held the biggest share in the market due to its robust healthcare infrastructure, technological advancements, and high prevalence of cardiovascular diseases. The region's well-established healthcare facilities, coupled with a strong emphasis on patient safety and outcomes, catalyze the demand for effective embolic protection strategies. Favorable reimbursement policies and a propensity for adopting innovative medical technologies further stimulate the adoption of embolic protection devices in diverse cardiovascular interventions.
Additionally, the presence of various key players in the region and ongoing research initiatives contribute to the continuous evolution of embolic protection technologies. With its combination of clinical need, economic factors, and technological readiness, North America has emerged as a leading regional market for embolic protection devices.
Competitive Landscape:
Key players in the embolic protection devices market have recently introduced noteworthy innovations to address critical challenges. They have unveiled advanced embolic protection devices with improved navigational capabilities, real-time monitoring features, and enhanced thrombus capture efficiency. These innovations aim to provide healthcare professionals with greater precision and insight during complex procedures like transcatheter interventions and carotid artery stenting. Furthermore, the leading players have invested in developing integrated systems that seamlessly integrate with existing procedural workflows, streamlining the process and minimizing procedural time. These advancements reflect the industry's commitment to refining embolic protection strategies and reinforcing patient safety, thereby solidifying their role as frontrunners in shaping the landscape of embolic protection technologies. We also expect the market to witness new entrants, consolidation of product portfolios, and increased collaborations among manufacturers to drive healthy competition within the embolic protection devices domain.The market research report has provided a comprehensive analysis of the competitive landscape in the market. Detailed profiles of all major companies have also been provided. Some of the key players in the market include:
- Abbott Laboratories
- Allium Medical Solutions Ltd.
- Boston Scientific Corporation
- Cardinal Health Inc.
- Contego Medical LLC
- Innovative Cardiovascular Solutions LLC
- Edwards Lifesciences Corporation
- Medtronic Inc.
- Silk Road Medical Inc.
Key Questions Answered in This Report
1. What was the size of the global embolic protection devices market in 2024?2. What is the expected growth rate of the global embolic protection devices market during 2025-2033?
3. What has been the impact of COVID-19 on the global embolic protection devices market?
4. What are the key factors driving the global embolic protection devices market?
5. What is the breakup of the global embolic protection devices market based on the product?
6. What is the breakup of the global embolic protection devices market based on the application?
7. What is the breakup of the global embolic protection devices market based on the procedure?
8. What is the breakup of the global embolic protection devices market based on the material?
9. What is the breakup of the global embolic protection devices market based on the usage?
10. What is the breakup of the global embolic protection devices market based on the end-user?
11. What are the key regions in the global embolic protection devices market?
12. Who are the key players/companies in the global embolic protection devices market?
Table of Contents
Companies Mentioned
- Abbott Laboratories
- Allium Medical Solutions Ltd.
- Boston Scientific Corporation
- Cardinal Health Inc.
- Contego Medical LLC
- Innovative Cardiovascular Solutions LLC
- Edwards Lifesciences Corporation
- Medtronic Inc.
- Silk Road Medical Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 117 |
| Published | August 2025 |
| Forecast Period | 2024 - 2033 |
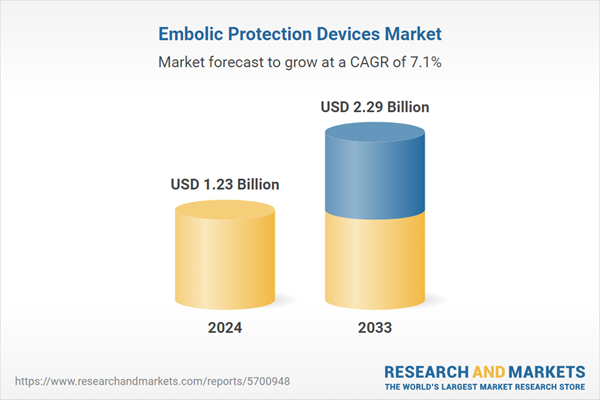
| Estimated Market Value ( USD | $ 1.23 Billion |
| Forecasted Market Value ( USD | $ 2.29 Billion |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |