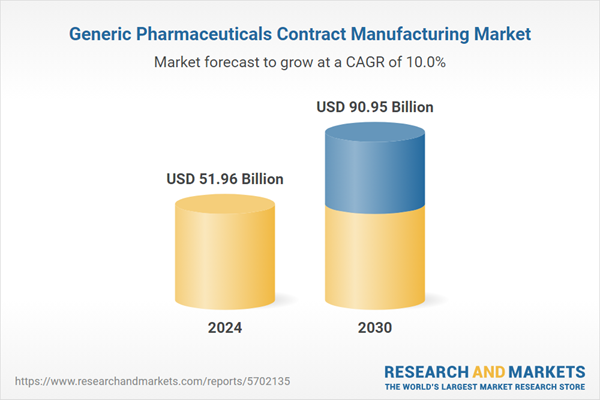
The global generic pharmaceuticals contract manufacturing market size is expected to reach USD 90.95 billion by 2030, registering a CAGR of 9.98% from 2025 to 2030. Cost-saving and time-saving benefits associated with the implementation of outsourcing is responsible for driving the industry. A significant number of people globally suffer from chronic diseases. For instance, the CDC states that 6 in 10 adults in the U.S. suffer from at least one chronic disease and 4 in 10 adults suffer from two or more chronic diseases. Chronic diseases are required to be treated for a long time. The high cost of medicines is increasing the demand for cost-effective generic drugs for the treatment of chronic diseases.
This is expected to support the industry's growth post-pandemic. There is an improvement in the regulatory approval of generic drugs. For instance, in 2021, the FDA approved 93 generic drugs, and by October 2022, the regulatory authority approved over 95 generic drugs. Such improvements are expected to have a positive impact on the manufacturing of generic drugs and; thus, support the industry growth. The Japanese government is constantly trying to improve the generic pharmaceuticals market in the country. The government is also taking measures to improve the supply of generics in the country and is also encouraging medical institutes to promote the use of generic drugs.
This is expected to improve CMO activities for generics in the coming years. Global spending on medicines is also on the rise. According to the data provided in a report published by IQVIA in April 2021, global spending on medicine is expected to increase in the next 4-5 years. The report states that global spending on medicine accounted for USD 1.26 trillion in 2020 and is going to reach USD 1,580-1,610 billion by 2025. This is also expected to improve the demand for generic drugs owing to their cost efficiency, thereby supporting the industry in growth.
This product will be delivered within 1-3 business days.
This is expected to support the industry's growth post-pandemic. There is an improvement in the regulatory approval of generic drugs. For instance, in 2021, the FDA approved 93 generic drugs, and by October 2022, the regulatory authority approved over 95 generic drugs. Such improvements are expected to have a positive impact on the manufacturing of generic drugs and; thus, support the industry growth. The Japanese government is constantly trying to improve the generic pharmaceuticals market in the country. The government is also taking measures to improve the supply of generics in the country and is also encouraging medical institutes to promote the use of generic drugs.
This is expected to improve CMO activities for generics in the coming years. Global spending on medicines is also on the rise. According to the data provided in a report published by IQVIA in April 2021, global spending on medicine is expected to increase in the next 4-5 years. The report states that global spending on medicine accounted for USD 1.26 trillion in 2020 and is going to reach USD 1,580-1,610 billion by 2025. This is also expected to improve the demand for generic drugs owing to their cost efficiency, thereby supporting the industry in growth.
Generic Pharmaceuticals Contract Manufacturing Market Report Highlights
- On the basis of drug type segment, the market is segmented into branded generics, and unbranded generics. In 2024, the branded generics segment dominated the market, accounting for a revenue share of 59.40%.
- On the basis of the product segment, the market is segmented to API, and Drug Product. API segment accounted for the largest market share in 2024.
- On the basis of route of administration segment, the market includes oral, parenteral, topical, and others. In 2024, the oral segment dominated the market, accounting for a revenue share of 63.73%.
- On the basis of application segment, the market includes oncology, immunology, antidiabetic, neurology, anticoagulants, cardiovascular, respiratory, pain, HIV antivirals, and others.
- North America accounted for the largest market share of 38.90% in 2024. This can be attributed to the growth of the pharmaceutical industries in the U.S. and Canada.
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This Report Addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 1-3 business days.
Table of Contents
Chapter 1. Research Methodology and Scope
1.1. Market Segmentation & Scope
1.2. Segment Definitions
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased Database
1.4.2. Internal Database
1.4.3. Secondary Sources
1.4.4. Primary Research
1.5. Information Or Data Analysis
1.5.1. Data Analysis Models
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity Flow Analysis
1.7.2. Parent Market Analysis
1.8. List Of Secondary Sources
1.9. List Of Abbreviations
1.10. Objectives
1.2. Segment Definitions
1.3. Research Methodology
1.4. Information Procurement
1.4.1. Purchased Database
1.4.2. Internal Database
1.4.3. Secondary Sources
1.4.4. Primary Research
1.5. Information Or Data Analysis
1.5.1. Data Analysis Models
1.6. Market Formulation & Validation
1.7. Model Details
1.7.1. Commodity Flow Analysis
1.7.2. Parent Market Analysis
1.8. List Of Secondary Sources
1.9. List Of Abbreviations
1.10. Objectives
Chapter 2. Executive Summary
2.1. Market Outlook
2.2. Segment Outlook
2.3. Competitive Insights
2.2. Segment Outlook
2.3. Competitive Insights
Chapter 3. Generic Pharmaceuticals Contract Manufacturing Market Variables, Trends & Scope
3.1. Market Lineage Outlook
3.1.1. Parent Market Outlook
3.1.2. Related/Ancillary Market Outlook
3.2. Market Dynamics
3.2.1. Market Driver Analysis
3.2.2. Market Restraint Analysis
3.3. Technological Advancements
3.4. Total volume of clinical trials (2024)
3.4.1. Total volume of clinical trials, by region
3.4.2. Total volume of clinical trials, by phase
3.4.3. Total volume of clinical trials, by application
3.5. Pricing Model Analysis
3.6. Market Analysis Tools
3.6.1. Porter’s Five Analysis
3.6.2. PESTEL by SWOT Analysis
3.6.3. COVID-19 Impact Analysis
3.1.1. Parent Market Outlook
3.1.2. Related/Ancillary Market Outlook
3.2. Market Dynamics
3.2.1. Market Driver Analysis
3.2.2. Market Restraint Analysis
3.3. Technological Advancements
3.4. Total volume of clinical trials (2024)
3.4.1. Total volume of clinical trials, by region
3.4.2. Total volume of clinical trials, by phase
3.4.3. Total volume of clinical trials, by application
3.5. Pricing Model Analysis
3.6. Market Analysis Tools
3.6.1. Porter’s Five Analysis
3.6.2. PESTEL by SWOT Analysis
3.6.3. COVID-19 Impact Analysis
Chapter 4. Generic Pharmaceuticals Contract Manufacturing Market: Drug Type Estimates & Trend Analysis
4.1. Generic Pharmaceuticals Contract Manufacturing Market, By Drug Type: Segment Dashboard
4.2. Generic Pharmaceuticals Contract Manufacturing Market, By Drug Type: Movement Analysis
4.3. Generic Pharmaceuticals Contract Manufacturing Market Estimates & Forecasts, By Drug Type, 2018 - 2030
4.4. Branded Generics
4.4.1. Branded Generics Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
4.5. Unbranded Generics
4.5.1. Unbranded Generics Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
4.2. Generic Pharmaceuticals Contract Manufacturing Market, By Drug Type: Movement Analysis
4.3. Generic Pharmaceuticals Contract Manufacturing Market Estimates & Forecasts, By Drug Type, 2018 - 2030
4.4. Branded Generics
4.4.1. Branded Generics Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
4.5. Unbranded Generics
4.5.1. Unbranded Generics Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 5. Generic Pharmaceuticals Contract Manufacturing Market: Product Estimates & Trend Analysis
5.1. Generic Pharmaceuticals Contract Manufacturing Market, By Product: Segment Dashboard
5.2. Generic Pharmaceuticals Contract Manufacturing Market, By Product: Movement Analysis
5.3. Generic Pharmaceuticals Contract Manufacturing Market Estimates & Forecasts, By Product, 2018 - 2030
5.4. API
5.4.1. API Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
5.5. Drug Product
5.5.1. Drug Product Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
5.2. Generic Pharmaceuticals Contract Manufacturing Market, By Product: Movement Analysis
5.3. Generic Pharmaceuticals Contract Manufacturing Market Estimates & Forecasts, By Product, 2018 - 2030
5.4. API
5.4.1. API Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
5.5. Drug Product
5.5.1. Drug Product Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 6. Generic Pharmaceuticals Contract Manufacturing Market: Route of Administration Estimates & Trend Analysis
6.1. Generic Pharmaceuticals Contract Manufacturing Market, By Route of Administration: Segment Dashboard
6.2. Generic Pharmaceuticals Contract Manufacturing Market, By Route of Administration: Movement Analysis
6.3. Generic Pharmaceuticals Contract Manufacturing Market Estimates & Forecasts, By Route of Administration, 2018 - 2030
6.4. Oral
6.4.1. Oral Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5. Parenteral
6.5.1. Parenteral Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.6. Topical
6.6.1. Topical Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.7. Others
6.7.1. Others Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.2. Generic Pharmaceuticals Contract Manufacturing Market, By Route of Administration: Movement Analysis
6.3. Generic Pharmaceuticals Contract Manufacturing Market Estimates & Forecasts, By Route of Administration, 2018 - 2030
6.4. Oral
6.4.1. Oral Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.5. Parenteral
6.5.1. Parenteral Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.6. Topical
6.6.1. Topical Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
6.7. Others
6.7.1. Others Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 7. Generic Pharmaceuticals Contract Manufacturing Market: Application Estimates & Trend Analysis
7.1. Generic Pharmaceuticals Contract Manufacturing Market, By Application: Segment Dashboard
7.2. Generic Pharmaceuticals Contract Manufacturing Market, By Application: Movement Analysis
7.3. Generic Pharmaceuticals Contract Manufacturing Market Estimates & Forecasts, By Application, 2018 - 2030
7.4. Oncology
7.4.1. Oncology Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.5. Immunology
7.5.1. Immunology Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.6. Antidiabetic
7.6.1. Antidiabetic Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.7. Neurology
7.7.1. Neurology Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.8. Anticoagulants
7.8.1. Anticoagulants Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.9. Cardiovascular
7.9.1. Cardiovascular Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.10. Respiratory
7.10.1. Respiratory Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.11. Pain
7.11.1. Pain Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.12. HIV Antivirals
7.12.1. HIV Antivirals Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.13. Others
7.13.1. Others Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.2. Generic Pharmaceuticals Contract Manufacturing Market, By Application: Movement Analysis
7.3. Generic Pharmaceuticals Contract Manufacturing Market Estimates & Forecasts, By Application, 2018 - 2030
7.4. Oncology
7.4.1. Oncology Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.5. Immunology
7.5.1. Immunology Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.6. Antidiabetic
7.6.1. Antidiabetic Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.7. Neurology
7.7.1. Neurology Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.8. Anticoagulants
7.8.1. Anticoagulants Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.9. Cardiovascular
7.9.1. Cardiovascular Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.10. Respiratory
7.10.1. Respiratory Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.11. Pain
7.11.1. Pain Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.12. HIV Antivirals
7.12.1. HIV Antivirals Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
7.13. Others
7.13.1. Others Market Revenue Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 8. Generic Pharmaceuticals Contract Manufacturing Market: Regional Estimates & Trend Analysis
8.1. Regional Market Share Analysis, 2024 & 2030
8.2. Regional Market Dashboard
8.3. Global Regional Market Snapshot
8.4. North America
8.4.1. North America Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.4.2. U.S
8.4.2.1. Key Country Dynamics
8.4.2.2. Competitive Scenario
8.4.2.3. Regulatory Framework
8.4.2.4. U.S. Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.4.3. Canada
8.4.3.1. Key Country Dynamics
8.4.3.2. Competitive Scenario
8.4.3.3. Regulatory Framework
8.4.3.4. Canada Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.4.4. Mexico
8.4.4.1. Key Country Dynamics
8.4.4.2. Competitive Scenario
8.4.4.3. Regulatory Framework
8.4.4.4. Mexico Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5. Europe
8.5.1. Europe Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.2. UK
8.5.2.1. Key Country Dynamics
8.5.2.2. Competitive Scenario
8.5.2.3. Regulatory Framework
8.5.2.4. UK Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.3. Germany
8.5.3.1. Key Country Dynamics
8.5.3.2. Competitive Scenario
8.5.3.3. Regulatory Framework
8.5.3.4. Germany Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.4. France
8.5.4.1. Key Country Dynamics
8.5.4.2. Competitive Scenario
8.5.4.3. Regulatory Framework
8.5.4.4. France Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.5. Italy
8.5.5.1. Key Country Dynamics
8.5.5.2. Competitive Scenario
8.5.5.3. Regulatory Framework
8.5.5.4. Italy Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.6. Spain
8.5.6.1. Key Country Dynamics
8.5.6.2. Competitive Scenario
8.5.6.3. Regulatory Framework
8.5.6.4. Spain Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.7. Denmark
8.5.7.1. Key Country Dynamics
8.5.7.2. Competitive Scenario
8.5.7.3. Regulatory Framework
8.5.7.4. Denmark Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.8. Sweden
8.5.8.1. Key Country Dynamics
8.5.8.2. Competitive Scenario
8.5.8.3. Regulatory Framework
8.5.8.4. Sweden Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.9. Norway
8.5.9.1. Key Country Dynamics
8.5.9.2. Competitive Scenario
8.5.9.3. Regulatory Framework
8.5.9.4. Norway Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6. Asia Pacific
8.6.1. Asia Pacific Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.2. Japan
8.6.2.1. Key Country Dynamics
8.6.2.2. Competitive Scenario
8.6.2.3. Regulatory Framework
8.6.2.4. Japan Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.3. China
8.6.3.1. Key Country Dynamics
8.6.3.2. Competitive Scenario
8.6.3.3. Regulatory Framework
8.6.3.4. China Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.4. India
8.6.4.1. Key Country Dynamics
8.6.4.2. Competitive Scenario
8.6.4.3. Regulatory Framework
8.6.4.4. India Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.5. Thailand
8.6.5.1. Key Country Dynamics
8.6.5.2. Competitive Scenario
8.6.5.3. Regulatory Framework
8.6.5.4. Thailand Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.6. South Korea
8.6.6.1. Key Country Dynamics
8.6.6.2. Competitive Scenario
8.6.6.3. Regulatory Framework
8.6.6.4. South Korea Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.7. Australia
8.6.7.1. Key Country Dynamics
8.6.7.2. Competitive Scenario
8.6.7.3. Regulatory Framework
8.6.7.4. Australia Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.7. Latin America
8.7.1. Latin America Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.7.2. Brazil
8.7.2.1. Key Country Dynamics
8.7.2.2. Competitive Scenario
8.7.2.3. Regulatory Framework
8.7.2.4. Brazil Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.7.3. Argentina
8.7.3.1. Key Country Dynamics
8.7.3.2. Competitive Scenario
8.7.3.3. Regulatory Framework
8.7.3.4. Argentina Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8. MEA
8.8.1. MEA Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.2. South Africa
8.8.2.1. Key Country Dynamics
8.8.2.2. Competitive Scenario
8.8.2.3. Regulatory Framework
8.8.2.4. South Africa Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.3. Saudi Arabia
8.8.3.1. Key Country Dynamics
8.8.3.2. Competitive Scenario
8.8.3.3. Regulatory Framework
8.8.3.4. Saudi Arabia Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.4. UAE
8.8.4.1. Key Country Dynamics
8.8.4.2. Competitive Scenario
8.8.4.3. Regulatory Framework
8.8.4.4. UAE Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.5. Kuwait
8.8.5.1. Key Country Dynamics
8.8.5.2. Competitive Scenario
8.8.5.3. Regulatory Framework
8.8.5.4. Kuwait Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.2. Regional Market Dashboard
8.3. Global Regional Market Snapshot
8.4. North America
8.4.1. North America Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.4.2. U.S
8.4.2.1. Key Country Dynamics
8.4.2.2. Competitive Scenario
8.4.2.3. Regulatory Framework
8.4.2.4. U.S. Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.4.3. Canada
8.4.3.1. Key Country Dynamics
8.4.3.2. Competitive Scenario
8.4.3.3. Regulatory Framework
8.4.3.4. Canada Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.4.4. Mexico
8.4.4.1. Key Country Dynamics
8.4.4.2. Competitive Scenario
8.4.4.3. Regulatory Framework
8.4.4.4. Mexico Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5. Europe
8.5.1. Europe Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.2. UK
8.5.2.1. Key Country Dynamics
8.5.2.2. Competitive Scenario
8.5.2.3. Regulatory Framework
8.5.2.4. UK Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.3. Germany
8.5.3.1. Key Country Dynamics
8.5.3.2. Competitive Scenario
8.5.3.3. Regulatory Framework
8.5.3.4. Germany Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.4. France
8.5.4.1. Key Country Dynamics
8.5.4.2. Competitive Scenario
8.5.4.3. Regulatory Framework
8.5.4.4. France Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.5. Italy
8.5.5.1. Key Country Dynamics
8.5.5.2. Competitive Scenario
8.5.5.3. Regulatory Framework
8.5.5.4. Italy Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.6. Spain
8.5.6.1. Key Country Dynamics
8.5.6.2. Competitive Scenario
8.5.6.3. Regulatory Framework
8.5.6.4. Spain Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.7. Denmark
8.5.7.1. Key Country Dynamics
8.5.7.2. Competitive Scenario
8.5.7.3. Regulatory Framework
8.5.7.4. Denmark Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.8. Sweden
8.5.8.1. Key Country Dynamics
8.5.8.2. Competitive Scenario
8.5.8.3. Regulatory Framework
8.5.8.4. Sweden Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.5.9. Norway
8.5.9.1. Key Country Dynamics
8.5.9.2. Competitive Scenario
8.5.9.3. Regulatory Framework
8.5.9.4. Norway Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6. Asia Pacific
8.6.1. Asia Pacific Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.2. Japan
8.6.2.1. Key Country Dynamics
8.6.2.2. Competitive Scenario
8.6.2.3. Regulatory Framework
8.6.2.4. Japan Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.3. China
8.6.3.1. Key Country Dynamics
8.6.3.2. Competitive Scenario
8.6.3.3. Regulatory Framework
8.6.3.4. China Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.4. India
8.6.4.1. Key Country Dynamics
8.6.4.2. Competitive Scenario
8.6.4.3. Regulatory Framework
8.6.4.4. India Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.5. Thailand
8.6.5.1. Key Country Dynamics
8.6.5.2. Competitive Scenario
8.6.5.3. Regulatory Framework
8.6.5.4. Thailand Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.6. South Korea
8.6.6.1. Key Country Dynamics
8.6.6.2. Competitive Scenario
8.6.6.3. Regulatory Framework
8.6.6.4. South Korea Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.6.7. Australia
8.6.7.1. Key Country Dynamics
8.6.7.2. Competitive Scenario
8.6.7.3. Regulatory Framework
8.6.7.4. Australia Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.7. Latin America
8.7.1. Latin America Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.7.2. Brazil
8.7.2.1. Key Country Dynamics
8.7.2.2. Competitive Scenario
8.7.2.3. Regulatory Framework
8.7.2.4. Brazil Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.7.3. Argentina
8.7.3.1. Key Country Dynamics
8.7.3.2. Competitive Scenario
8.7.3.3. Regulatory Framework
8.7.3.4. Argentina Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8. MEA
8.8.1. MEA Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.2. South Africa
8.8.2.1. Key Country Dynamics
8.8.2.2. Competitive Scenario
8.8.2.3. Regulatory Framework
8.8.2.4. South Africa Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.3. Saudi Arabia
8.8.3.1. Key Country Dynamics
8.8.3.2. Competitive Scenario
8.8.3.3. Regulatory Framework
8.8.3.4. Saudi Arabia Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.4. UAE
8.8.4.1. Key Country Dynamics
8.8.4.2. Competitive Scenario
8.8.4.3. Regulatory Framework
8.8.4.4. UAE Market Estimates and Forecasts, 2018 - 2030 (USD Million)
8.8.5. Kuwait
8.8.5.1. Key Country Dynamics
8.8.5.2. Competitive Scenario
8.8.5.3. Regulatory Framework
8.8.5.4. Kuwait Market Estimates and Forecasts, 2018 - 2030 (USD Million)
Chapter 9. Competitive Landscape
9.1. Market Participant Categorization
9.2. Company Market Share Analysis, 2024
9.3. Company Profiles
9.3.1. The Lubrizol Corporation
9.3.1.1. Company Overview
9.3.1.2. Financial Performance
9.3.1.3. Service Benchmarking
9.3.1.4. Strategic Initiatives
9.3.2. Jubilant Generics Ltd.
9.3.2.1. Company Overview
9.3.2.2. Financial Performance
9.3.2.3. Service Benchmarking
9.3.2.4. Strategic Initiatives
9.3.3. Recipharm AB
9.3.3.1. Company Overview
9.3.3.2. Financial Performance
9.3.3.3. Service Benchmarking
9.3.3.4. Strategic Initiatives
9.3.4. Siegfried Holding AG
9.3.4.1. Company Overview
9.3.4.2. Financial Performance
9.3.4.3. Service Benchmarking
9.3.4.4. Strategic Initiatives
9.3.5. Aurobindo Pharma
9.3.5.1. Company Overview
9.3.5.2. Financial Performance
9.3.5.3. Service Benchmarking
9.3.5.4. Strategic Initiatives
9.3.6. Cambrex Corp.
9.3.6.1. Company Overview
9.3.6.2. Financial Performance
9.3.6.3. Service Benchmarking
9.3.6.4. Strategic Initiatives
9.3.7. Alcami Corp., Inc.
9.3.7.1. Company Overview
9.3.7.2. Financial Performance
9.3.7.3. Service Benchmarking
9.3.7.4. Strategic Initiatives
9.3.8. Catalent, Inc.
9.3.8.1. Company Overview
9.3.8.2. Financial Performance
9.3.8.3. Service Benchmarking
9.3.8.4. Strategic Initiatives
9.3.9. Acme Generics Pvt Ltd.
9.3.9.1. Company Overview
9.3.9.2. Financial Performance
9.3.9.3. Service Benchmarking
9.3.9.4. Strategic Initiatives
9.3.10. Syngene International Ltd.
9.3.10.1. Company Overview
9.3.10.2. Financial Performance
9.3.10.3. Service Benchmarking
9.3.10.4. Strategic Initiatives
9.3.11. Pfizer CentreOne
9.3.11.1. Company Overview
9.3.11.2. Financial Performance
9.3.11.3. Service Benchmarking
9.3.11.4. Strategic Initiatives
9.3.12. Curia Global, Inc.
9.3.12.1. Company Overview
9.3.12.2. Financial Performance
9.3.12.3. Service Benchmarking
9.3.12.4. Strategic Initiatives
9.3.13. Metric Contract Services
9.3.13.1. Company Overview
9.3.13.2. Financial Performance
9.3.13.3. Service Benchmarking
9.3.13.4. Strategic Initiatives
9.2. Company Market Share Analysis, 2024
9.3. Company Profiles
9.3.1. The Lubrizol Corporation
9.3.1.1. Company Overview
9.3.1.2. Financial Performance
9.3.1.3. Service Benchmarking
9.3.1.4. Strategic Initiatives
9.3.2. Jubilant Generics Ltd.
9.3.2.1. Company Overview
9.3.2.2. Financial Performance
9.3.2.3. Service Benchmarking
9.3.2.4. Strategic Initiatives
9.3.3. Recipharm AB
9.3.3.1. Company Overview
9.3.3.2. Financial Performance
9.3.3.3. Service Benchmarking
9.3.3.4. Strategic Initiatives
9.3.4. Siegfried Holding AG
9.3.4.1. Company Overview
9.3.4.2. Financial Performance
9.3.4.3. Service Benchmarking
9.3.4.4. Strategic Initiatives
9.3.5. Aurobindo Pharma
9.3.5.1. Company Overview
9.3.5.2. Financial Performance
9.3.5.3. Service Benchmarking
9.3.5.4. Strategic Initiatives
9.3.6. Cambrex Corp.
9.3.6.1. Company Overview
9.3.6.2. Financial Performance
9.3.6.3. Service Benchmarking
9.3.6.4. Strategic Initiatives
9.3.7. Alcami Corp., Inc.
9.3.7.1. Company Overview
9.3.7.2. Financial Performance
9.3.7.3. Service Benchmarking
9.3.7.4. Strategic Initiatives
9.3.8. Catalent, Inc.
9.3.8.1. Company Overview
9.3.8.2. Financial Performance
9.3.8.3. Service Benchmarking
9.3.8.4. Strategic Initiatives
9.3.9. Acme Generics Pvt Ltd.
9.3.9.1. Company Overview
9.3.9.2. Financial Performance
9.3.9.3. Service Benchmarking
9.3.9.4. Strategic Initiatives
9.3.10. Syngene International Ltd.
9.3.10.1. Company Overview
9.3.10.2. Financial Performance
9.3.10.3. Service Benchmarking
9.3.10.4. Strategic Initiatives
9.3.11. Pfizer CentreOne
9.3.11.1. Company Overview
9.3.11.2. Financial Performance
9.3.11.3. Service Benchmarking
9.3.11.4. Strategic Initiatives
9.3.12. Curia Global, Inc.
9.3.12.1. Company Overview
9.3.12.2. Financial Performance
9.3.12.3. Service Benchmarking
9.3.12.4. Strategic Initiatives
9.3.13. Metric Contract Services
9.3.13.1. Company Overview
9.3.13.2. Financial Performance
9.3.13.3. Service Benchmarking
9.3.13.4. Strategic Initiatives
List of Tables
Table 1. List of Secondary Sources
Table 2. List of Abbreviations
Table 3. Global Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 4. Global Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 5. Global Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 6. Global Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 7. Global Generic Pharmaceuticals Contract Manufacturing Market, by Region, 2018 - 2030 (USD Million)
Table 8. North America Generic Pharmaceuticals Contract Manufacturing Market, by Country, 2018 - 2030 (USD Million)
Table 9. North America Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 10. North America Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 11. North America Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 12. North America Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 13. U.S. Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 14. U.S. Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 15. U.S. Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 16. U.S. Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 17. Canada Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 18. Canada Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 19. Canada Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 20. Canada Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 21. Mexico Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 22. Mexico Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 23. Mexico Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 24. Mexico Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 25. Europe Generic Pharmaceuticals Contract Manufacturing Market, by Country, 2018 - 2030 (USD Million)
Table 26. Europe Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 27. Europe Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 28. Europe Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 29. Europe Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 30. UK Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 31. UK Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 32. UK Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 33. UK Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 34. Germany Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 35. Germany Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 36. Germany Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 37. Germany Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 38. France Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 39. France Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 40. France Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 41. France Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 42. Italy Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 43. Italy Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 44. Italy Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 45. Italy Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 46. Spain Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 47. Spain Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 48. Spain Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 49. Spain Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 50. Denmark Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 51. Denmark Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 52. Denmark Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 53. Denmark Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 54. Sweden Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 55. Sweden Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 56. Sweden Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 57. Sweden Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 58. Norway Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 59. Norway Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 60. Norway Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 61. Norway Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 62. Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market, by Country, 2018 - 2030 (USD Million)
Table 63. Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 64. Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 65. Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 66. Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 67. Japan Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 68. Japan Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 69. Japan Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 70. Japan Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 71. China Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 72. China Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 73. China Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 74. China Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 75. India Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 76. India Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 77. India Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 78. India Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 79. Thailand Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 80. Thailand Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 81. Thailand Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 82. Thailand Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 83. South Korea Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 84. South Korea Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 85. South Korea Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 86. South Korea Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 87. Australia Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 88. Australia Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 89. Australia Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 90. Australia Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 91. Latin America Generic Pharmaceuticals Contract Manufacturing Market, by Country, 2018 - 2030 (USD Million)
Table 92. Latin America Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 93. Latin America Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 94. Latin America Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 95. Latin America Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 96. Brazil Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 97. Brazil Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 98. Brazil Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 99. Brazil Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 100. Argentina Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 101. Argentina Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 102. Argentina Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 103. Argentina Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 104. Middle East & Africa Generic Pharmaceuticals Contract Manufacturing Market, by Country, 2018 - 2030 (USD Million)
Table 105. Middle East & Africa Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 106. Middle East & Africa Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 107. Middle East & Africa Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 108. Middle East & Africa Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 109. South Africa Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 110. South Africa Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 111. South Africa Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 112. South Africa Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 113. Saudi Arabia Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 114. Saudi Arabia Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 115. Saudi Arabia Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 116. Saudi Arabia Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 117. UAE Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 118. UAE Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 119. UAE Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 120. UAE Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 121. Kuwait Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 122. Kuwait Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 123. Kuwait Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 124. Kuwait Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 2. List of Abbreviations
Table 3. Global Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 4. Global Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 5. Global Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 6. Global Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 7. Global Generic Pharmaceuticals Contract Manufacturing Market, by Region, 2018 - 2030 (USD Million)
Table 8. North America Generic Pharmaceuticals Contract Manufacturing Market, by Country, 2018 - 2030 (USD Million)
Table 9. North America Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 10. North America Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 11. North America Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 12. North America Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 13. U.S. Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 14. U.S. Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 15. U.S. Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 16. U.S. Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 17. Canada Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 18. Canada Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 19. Canada Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 20. Canada Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 21. Mexico Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 22. Mexico Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 23. Mexico Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 24. Mexico Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 25. Europe Generic Pharmaceuticals Contract Manufacturing Market, by Country, 2018 - 2030 (USD Million)
Table 26. Europe Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 27. Europe Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 28. Europe Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 29. Europe Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 30. UK Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 31. UK Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 32. UK Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 33. UK Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 34. Germany Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 35. Germany Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 36. Germany Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 37. Germany Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 38. France Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 39. France Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 40. France Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 41. France Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 42. Italy Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 43. Italy Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 44. Italy Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 45. Italy Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 46. Spain Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 47. Spain Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 48. Spain Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 49. Spain Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 50. Denmark Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 51. Denmark Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 52. Denmark Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 53. Denmark Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 54. Sweden Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 55. Sweden Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 56. Sweden Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 57. Sweden Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 58. Norway Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 59. Norway Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 60. Norway Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 61. Norway Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 62. Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market, by Country, 2018 - 2030 (USD Million)
Table 63. Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 64. Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 65. Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 66. Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 67. Japan Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 68. Japan Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 69. Japan Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 70. Japan Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 71. China Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 72. China Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 73. China Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 74. China Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 75. India Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 76. India Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 77. India Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 78. India Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 79. Thailand Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 80. Thailand Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 81. Thailand Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 82. Thailand Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 83. South Korea Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 84. South Korea Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 85. South Korea Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 86. South Korea Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 87. Australia Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 88. Australia Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 89. Australia Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 90. Australia Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 91. Latin America Generic Pharmaceuticals Contract Manufacturing Market, by Country, 2018 - 2030 (USD Million)
Table 92. Latin America Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 93. Latin America Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 94. Latin America Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 95. Latin America Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 96. Brazil Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 97. Brazil Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 98. Brazil Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 99. Brazil Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 100. Argentina Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 101. Argentina Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 102. Argentina Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 103. Argentina Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 104. Middle East & Africa Generic Pharmaceuticals Contract Manufacturing Market, by Country, 2018 - 2030 (USD Million)
Table 105. Middle East & Africa Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 106. Middle East & Africa Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 107. Middle East & Africa Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 108. Middle East & Africa Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 109. South Africa Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 110. South Africa Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 111. South Africa Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 112. South Africa Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 113. Saudi Arabia Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 114. Saudi Arabia Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 115. Saudi Arabia Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 116. Saudi Arabia Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 117. UAE Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 118. UAE Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 119. UAE Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 120. UAE Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
Table 121. Kuwait Generic Pharmaceuticals Contract Manufacturing Market, by Drug Type, 2018 - 2030 (USD Million)
Table 122. Kuwait Generic Pharmaceuticals Contract Manufacturing Market, by Product, 2018 - 2030 (USD Million)
Table 123. Kuwait Generic Pharmaceuticals Contract Manufacturing Market, by Route of Administration, 2018 - 2030 (USD Million)
Table 124. Kuwait Generic Pharmaceuticals Contract Manufacturing Market, by Application, 2018 - 2030 (USD Million)
List of Figures
Fig. 1 Market research process
Fig. 2 Information procurement
Fig. 3 Primary research pattern
Fig. 4 Market research approaches
Fig. 5 Market formulation & validation
Fig. 6 Commodity flow analysis
Fig. 7 Value-chain-based sizing & forecasting
Fig. 8 QFD model sizing & forecasting
Fig. 9 Bottom-up approach
Fig. 10 Market snapshot
Fig. 11 Segment snapshot 1
Fig. 12 Segment snapshot 2
Fig. 13 Competitive landscape snapshot
Fig. 14 Parent market outlook, 2024 (USD Billion)
Fig. 15 Ancillary market outlook, 2024 (USD Billion)
Fig. 16 Generic Pharmaceuticals Contract Manufacturing Market dynamics
Fig. 17 Porter’s five forces analysis
Fig. 18 PESTEL analysis
Fig. 19 Generic Pharmaceuticals Contract Manufacturing Market: Drug Type outlook and key takeaways
Fig. 20 Generic Pharmaceuticals Contract Manufacturing Market: Drug Type movement analysis
Fig. 21 Branded Generics market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 22 Unbranded Generics market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 23 Generic Pharmaceuticals Contract Manufacturing Market: Product outlook and key takeaways
Fig. 24 Generic Pharmaceuticals Contract Manufacturing Market: Product movement analysis
Fig. 25 API market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 26 Drug Product market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 27 Generic Pharmaceuticals Contract Manufacturing Market: Route of Administration outlook and key takeaways
Fig. 28 Generic Pharmaceuticals Contract Manufacturing Market: Route of Administration movement analysis
Fig. 29 Oral market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 30 Parenteral market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 31 Topical market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 32 Others market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 33 Generic Pharmaceuticals Contract Manufacturing Market: Application outlook and key takeaways
Fig. 34 Generic Pharmaceuticals Contract Manufacturing Market: Application movement analysis
Fig. 35 Oncology market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 36 Immunology market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 37 Antidiabetic market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 38 Neurology market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 39 Anticoagulants market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 40 Cardiovascular market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 41 Respiratory market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 42 Pain market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 43 HIV Antivirals market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 44 Others market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 45 Regional Marketplace: Key Takeaways
Fig. 46 North America Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 47 Key country dynamics
Fig. 48 U.S. Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 49 Key country dynamics
Fig. 50 Canada Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 51 Key country dynamics
Fig. 52 Mexico Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 53 Europe Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 54 Key country dynamics
Fig. 55 UK Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 56 Key country dynamics
Fig. 57 Germany Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 58 Key country dynamics
Fig. 59 France Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 60 Key country dynamics
Fig. 61 Italy Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 62 Key country dynamics
Fig. 63 Spain Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 64 Key country dynamics
Fig. 65 Denmark outsourcing market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 66 Key country dynamics
Fig. 67 Sweden outsourcing market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 68 Key country dynamics
Fig. 69 Norway outsourcing market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 70 Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 71 Key country dynamics
Fig. 72 Japan Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 73 Key country dynamics
Fig. 74 China Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 75 Key country dynamics
Fig. 76 India Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 77 Key country dynamics
Fig. 78 Thailand Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 79 Key country dynamics
Fig. 80 South Korea Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 81 Key country dynamics
Fig. 82 Australia Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 83 Latin America Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 84 Key country dynamics
Fig. 85 Brazil Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 86 Key country dynamics
Fig. 87 Argentina Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 88 MEA Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 89 Key country dynamics
Fig. 90 South Africa Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 91 Fig. Key country dynamics
Fig. 92 Saudi Arabia Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 93 Key country dynamics
Fig. 94 UAE Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 95 Key country dynamics
Fig. 96 Kuwait Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 97 Key company categorization
Fig. 98 Service heat map analysis
Fig. 99 Strategic framework
Fig. 2 Information procurement
Fig. 3 Primary research pattern
Fig. 4 Market research approaches
Fig. 5 Market formulation & validation
Fig. 6 Commodity flow analysis
Fig. 7 Value-chain-based sizing & forecasting
Fig. 8 QFD model sizing & forecasting
Fig. 9 Bottom-up approach
Fig. 10 Market snapshot
Fig. 11 Segment snapshot 1
Fig. 12 Segment snapshot 2
Fig. 13 Competitive landscape snapshot
Fig. 14 Parent market outlook, 2024 (USD Billion)
Fig. 15 Ancillary market outlook, 2024 (USD Billion)
Fig. 16 Generic Pharmaceuticals Contract Manufacturing Market dynamics
Fig. 17 Porter’s five forces analysis
Fig. 18 PESTEL analysis
Fig. 19 Generic Pharmaceuticals Contract Manufacturing Market: Drug Type outlook and key takeaways
Fig. 20 Generic Pharmaceuticals Contract Manufacturing Market: Drug Type movement analysis
Fig. 21 Branded Generics market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 22 Unbranded Generics market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 23 Generic Pharmaceuticals Contract Manufacturing Market: Product outlook and key takeaways
Fig. 24 Generic Pharmaceuticals Contract Manufacturing Market: Product movement analysis
Fig. 25 API market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 26 Drug Product market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 27 Generic Pharmaceuticals Contract Manufacturing Market: Route of Administration outlook and key takeaways
Fig. 28 Generic Pharmaceuticals Contract Manufacturing Market: Route of Administration movement analysis
Fig. 29 Oral market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 30 Parenteral market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 31 Topical market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 32 Others market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 33 Generic Pharmaceuticals Contract Manufacturing Market: Application outlook and key takeaways
Fig. 34 Generic Pharmaceuticals Contract Manufacturing Market: Application movement analysis
Fig. 35 Oncology market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 36 Immunology market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 37 Antidiabetic market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 38 Neurology market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 39 Anticoagulants market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 40 Cardiovascular market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 41 Respiratory market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 42 Pain market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 43 HIV Antivirals market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 44 Others market estimates and forecast, 2018 - 2030 (USD Million)
Fig. 45 Regional Marketplace: Key Takeaways
Fig. 46 North America Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 47 Key country dynamics
Fig. 48 U.S. Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 49 Key country dynamics
Fig. 50 Canada Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 51 Key country dynamics
Fig. 52 Mexico Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 53 Europe Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 54 Key country dynamics
Fig. 55 UK Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 56 Key country dynamics
Fig. 57 Germany Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 58 Key country dynamics
Fig. 59 France Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 60 Key country dynamics
Fig. 61 Italy Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 62 Key country dynamics
Fig. 63 Spain Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 64 Key country dynamics
Fig. 65 Denmark outsourcing market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 66 Key country dynamics
Fig. 67 Sweden outsourcing market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 68 Key country dynamics
Fig. 69 Norway outsourcing market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 70 Asia Pacific Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 71 Key country dynamics
Fig. 72 Japan Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 73 Key country dynamics
Fig. 74 China Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 75 Key country dynamics
Fig. 76 India Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 77 Key country dynamics
Fig. 78 Thailand Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 79 Key country dynamics
Fig. 80 South Korea Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 81 Key country dynamics
Fig. 82 Australia Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 83 Latin America Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 84 Key country dynamics
Fig. 85 Brazil Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 86 Key country dynamics
Fig. 87 Argentina Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 88 MEA Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 89 Key country dynamics
Fig. 90 South Africa Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 91 Fig. Key country dynamics
Fig. 92 Saudi Arabia Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 93 Key country dynamics
Fig. 94 UAE Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 95 Key country dynamics
Fig. 96 Kuwait Generic Pharmaceuticals Contract Manufacturing Market estimates and forecasts, 2018 - 2030 (USD Million)
Fig. 97 Key company categorization
Fig. 98 Service heat map analysis
Fig. 99 Strategic framework
Companies Mentioned
- Jubilant Generics Ltd.
- Recipharm AB
- Siegfried Holding AG
- Aurobindo Pharma
- Cambrex Corp.
- Alcami Corp., Inc.
- Catalent, Inc.
- Acme Generics Pvt Ltd.
- Syngene International Ltd.
- Pfizer CentreOne
- Curia Global, Inc.
- Metric Contract Services
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 150 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 51.96 Billion |
| Forecasted Market Value ( USD | $ 90.95 Billion |
| Compound Annual Growth Rate | 9.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |