Electroencephalography devices are utilized in monitoring and reviewing the electrical signals generated by the brain. These devices have electrodes that are attached to the scalp to register electrical activity. The outcomes from these devices are used in monitoring patients under anesthesia and those suffering from sleep disorders. The devices also aid in diagnosing various neurodegenerative brain disorders.
These devices are mainly used in diagnostic laboratories, hospitals, and ambulatory surgery centers and include EEG biofeedback machines or EEG neurofeedback machines. During the electroencephalography (EEG) of the brain, the electrogram recording the electrical activity is generated. The brain waves detected by the EEG device represent the pyramidal neurons’ postsynaptic potentials in the allocortex and neocortex. The EEG method is non-invasive as it involves attaching the electrodes all along the scalp with some adhesive.
The placement of these electrodes is governed by the 10-20 system or the international 10-20 system. The interpretation of brain wave recordings for clinical use is commonly done by quantitative or visual analysis of EEG recordings. The electrical activity in the brain produces minute voltages that are picked by the EEG devices.
Most EEG equipment comes with amplifiers, which help in the easy recognition of minute voltages and thus, help in producing accurate recordings. The transmission of ionic currents, which occur when the brain receives and processes information, from and among neurons brings changes in the voltages. These are then captured by the EEG devices. Besides amplifiers, EEG devices also contain filters, electrodes, and a converter that converts signals from analog to digital.
COVID-19 Impact Analysis
During the pandemic, an increase in the number of people suffering from sleep disorders and problems was recorded. This increase was because the pandemic worsened the working situation with most people working beyond the standard office hours. The lockdown restricted the hospitals and other healthcare facilities for admission of uninfected cases. This caused high cancellation rates of appointments and surgeries. Most infected patients with underlying brain disorders needed EEG devices for regular monitoring and thus, this maintained the demand of these devices in hospital and homecare settings. But other than that, the overall demand for the devices suffered negatively. Therefore, the COVID-19 pandemic had a negative impact on the electroencephalography devices market.Market Growth Factors
Rising prevalence of neurovascular disorders
Lifestyle habits, genetic disorders, and certain chronic conditions propagate the incidences of neurovascular diseases. These are the diseases that are related to the brain, spine, and connecting nerves, and significantly impair and hinder their functioning. In the past few years, these diseases have become dominant. The most commonly found neurovascular diseases include stroke, brain tumors, and ischemic strokes. Sedentary lifestyles and a lack of exercise hinder the optimal intake of oxygen.Increasing proportion of the aging population
Most recent research has extrapolated the population numbers from countries and the world to quantify the rise of the aging population. Subsequently, the outcomes have established this age group to be one of the most prominent social transformations. As per the WHO, one in six people in the world will be over 60 years of age by 2030, and the population would increase to around 1.4 billion. And in 2050, the aging population would be almost double of what it was in 2020.Market Restraining Factors
Low spatial resolution and less meaningful results
While EEGs offer a high temporal resolution, they deliver subpar results for spatial resolution. Even the data produced through the temporal resolution is inaccurate oftentimes and as such, can be considered less meaningful. Other techniques are able to display the active regions of the brain, while EEG necessitates the need for intense interpretation that would conclude and hypothesize the active regions for a specific response.Type Outlook
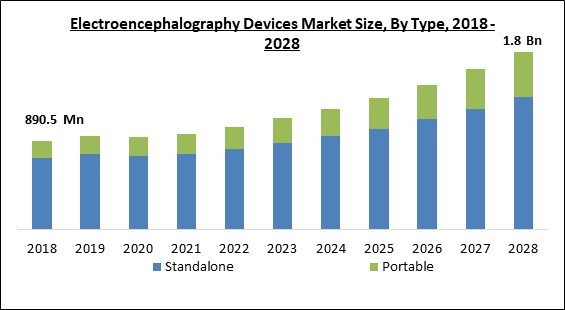
On the basis of type, the electroencephalography devices market is bifurcated into standalone devices and portable devices. The standalone segment acquired the largest revenue share in the electroencephalography devices market in 2021. Standalone EEG devices are implanted or fixed at a specific place and are mainly used in hospital settings. These systems include more software and hardware peripherals that allow the proper diagnosis of brain disorders. The applications of standalone EEG devices in critical care and intensive care units have made them the most common EEG device usage.End-Use Outlook
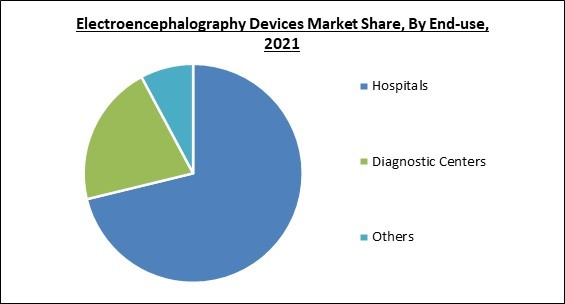
Based on end-use, the electroencephalography devices market is divided into hospitals, diagnostics centers, and others. The diagnostics segment garnered a prominent revenue share in the electroencephalography devices market in 2021. The growing awareness of people towards the impact of cognitive inactivity and brain disorders in daily life has increased the adoption of diagnostic services. As a consequence, the density of diagnostics centers has increased in most developed nations which in turn is propelling the demand for EEG devices and systems.Product Outlook
Based on product, the electroencephalography devices market is categorized into 8-channel EEG, 21-channel EEG, 25-channel EEG, 32-channel EEG, 40-channel EEG, and multi-channel EEG. The 32-channel EEG segment procured the highest revenue share in the electroencephalography devices market in 2021. The growth of the segment is attributable to the dominant use of 32-channel EEG in the diagnosis of various brain disorders. The increased efficiency of this product with 32 channels has made it an ideal EEG device, and as such many healthcare practitioners prefer the machine for regular monitoring and studies.Regional Outlook
On the basis of region, the electroencephalography devices market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment procured the highest revenue share in the electroencephalography devices market in 2021. The growing incidence of neurodegenerative disorders and various other sleep and brain disorders is advancing the use of EEG devices in the region, which is thereby boosting the growth of the segment. The availability of advanced healthcare infrastructure and convenient diagnostic facilities have influenced more individuals to increase their efforts in taking care of themselves.The market research report covers the analysis of key stakeholders of the market. Key companies profiled in the report include Medtronic PLC, Compumedics Limited, Natus Medical Incorporated (Archimed SAS), Nihon Kohden Corporation, Cadwell Industries, Inc., Neurowave Systems, Inc., Magstim EGI (Magstim, Inc.) and Noraxon U.S.A., Inc.
Strategies Deployed in Electroencephalography Devices Market
- Oct-2022: Nihon Kohden America, a subsidiary of Nihon Kohden Corporation, announced the launch of Life Scope® G7 patient monitor, an advanced bedside monitor across United States. Life Scope® G7 patient monitor comes with multiple features which includes detailed visuals, expanded data integration seamless workflow to benefit all.
- Oct-2022: Nihon Kohden introduced Smart Cable™ NMT Pod and disposable electrode, a device with the Smart Cable technology. The product lets clinicians to specifically monitor the patient’s depth of paralysis at the time of surgery when a neuromuscular blockade agent (NMBA), both depolarizing or non-depolarizing, is delivered to the patient.2020-Feb: Compumedics Limited recieved an FDA approval for Orion LifeSpan™ Magnetoencephalography (MEG) single Dewar system, a technique of neuroimaging to map brain activity. This Approval allows usage of MEG devices for clinical routine, primarily for pre-surgical brain function mapping and epilepsy.
- Aug-2022: Medtronic came into partnership with BioIntelliSense,a clinical intelligence and continuous health monitoring company. Under this partnership, Medtronic would support connected, continous care from in-hospital to home and broaden its presence to help additional patients in more locations.
- Sep-2021: NeuroWave Systems Inc. announced approval of FDA for NeuroSENSE® NS-901 Monitor, a brain function monitor of new genration. The product delivers high discrimination between unconsciousness and consciousness, quick response, linear response to increasingly deep anesthesia and individual anesthetic depth index for each brain side.
- Feb-2021: Cadwell Industries, Inc. completed the acquisition of Sleep Diagnostic Sensor's SleepMate line from Ambu A/S, a company engaged in delopment and producttion of diagnostic and life-supporting equipment and single-use endoscopy solutions. Through this acquisition, Cadwell Industries delivers SleepMate sensors to customers dependent on durablity, detection sensitivity and comfort of patient.
- Apr-2020: Nihon Kohden launched Premium Disposable Gold Cup EEG Electrode, a disposable electroencephalogram (EEG) electrode. The product helps healthcare providers contain cross-contamination between patients while keeping high-quality EEG signals. Additionally, Premium Disposable Gold Cup EEG Electrodes can help safeguard both healthcare workers and patients.
- Jun-2019: Nihon Kohden unveiled VitalEEG, a wireless electroencephalogram (EEG) headset. The VitalEEG supports clinicians to assess brain health rapidly and find further steps for the treatment of an unconscious patient.
- May-2019: Nihon Kohden released the Neuropack S3 electrodiagnostic (EDX) system, a multimodality platform for procedures of neurodiagnostic. This launch is aimed to be adaptable enough for usage in both private practices and hospitals to perform evoked potentials, nerve conduction studies, electromyography and other diagnostic procedures.
- Feb-2019: Medtronic got an FDA approval of Accurian RF ablation platform, a new device appropriate for pulsed, enhanced and standard procedures. The FDA approval enhances treatment of pain by offering choices to help patients during the time of care.
Scope of the Study
By Type
- Standalone
- Portable
By End-user
- Hospitals
- Diagnostic Centers
- Others
By Product
- 32-channel
- 25-channel
- 40-channel
- 21-channel
- 8-channel
- Multichannel
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Medtronic PLC
- Compumedics Limited
- Natus Medical Incorporated (Archimed SAS)
- Nihon Kohden Corporation
- Cadwell Industries, Inc.
- Neurowave Systems, Inc.
- Magstim EGI (Magstim, Inc.)
- Noraxon U.S.A., Inc.
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Medtronic PLC
- Compumedics Limited
- Natus Medical Incorporated (Archimed SAS)
- Nihon Kohden Corporation
- Cadwell Industries, Inc.
- Neurowave Systems, Inc.
- Magstim EGI (Magstim, Inc.)
- Noraxon U.S.A., Inc.