ENT refers to the ear, nose, and throat, and the term "ENT devices"refers to the equipment used to treat these organs. Doctors use these tools to treat illnesses affecting the ear, nose, and throat. There are several ENT products in the market that are made to treat issues with the ear, nose, and throat, as well as other head and neck structures.
Aural forceps, speculums, tongue depressors, nasal forceps, and laryngoscopes are a few of the more common devices utilized. Otolaryngology is the treatment and diagnosis of disorders of the sinuses, larynx, oral cavity, upper pharynx (mouth and throat), and associated tissues of the head and neck in both adults and children.
The ears, nose, throat, face, and neck are typically examined as part of a comprehensive ENT examination. Audiometric testing is carried out when a person has symptoms such as ringing in the ears, hearing loss, or balance issues. It is conducted using an audiometer in a soundproof room.
An audiometer is a device that assesses hearing acuity and includes a hardware unit attached to a set of headphones and a feedback button for the patient to record their comments. The quietest noises one can hear at every frequency, in each ear, are recorded by an audiologist and plotted as an "audiogram"on a graph. Tests using audiometry can determine if the patient has conductive or sensorineural hearing loss (injury to the nerve or cochlea).
COVID-19 Impact Analysis
The COVID-19 outbreak has caused delays in clinical operations. This may have a limited detrimental effect on the market expansion. For instance, leading ENT device manufacturers have reported delays in clinical trials. While the ENT device industry was negatively impacted by COVID-19 due to postponed or canceled operations. Cases with benign diseases, orthopedic conditions, or ENT issues were the ones that were most likely to be delayed or postponed. The hearing care sector depends heavily on close client interaction. In clinics and audiology shops, close human contact was discouraged or necessitated additional precautions as a result of the government's strict preventive measures.Market Growth Factors
Growth Opportunities In Developing Nations
For market players in ENT devices, emerging economies like Latin America, China, India, and nations provide considerable growth prospects. People in developing nations are now capable to buy ENT devices with cutting-edge technology, which is boosting the market's expansion. In addition, a number of prospects for the expansion of this market exist in emerging nations due to factors like laxer regulatory standards, growing urbanization, better healthcare facilities, increasing disposable income, and an ageing older population.Advancement In Technologies
Technology improvements in ENT devices and hearing aids will drive the market's expansion. Modern hearing aids come in small sizes and are attached to the ear through thin, invisible tubes. Miniaturization reduces the social stigma associated with hearing loss in adults and teenagers, which is beneficial for the market's growth. Additionally, some hearing aids have artificial intelligence (AI) built in that gives users access to a deep neural network for sound processingMarket Restraining Factors
Rising Pricing Pressure An Obstacle To Market Growth
Over the past few years, healthcare systems have made cost containment one of their top goals. Price regulations, bidding, competitive rates, tender processes, insurance and payment policies, comparative efficacy assessments of medications, technology reviews, and managed-care agreements are all used to achieve this. In response to a growing desire to cut healthcare costs, healthcare providers have partnered with group purchasing organizations and integrated medical networks that negotiate for the bulk purchase of medical equipment.Product Outlook
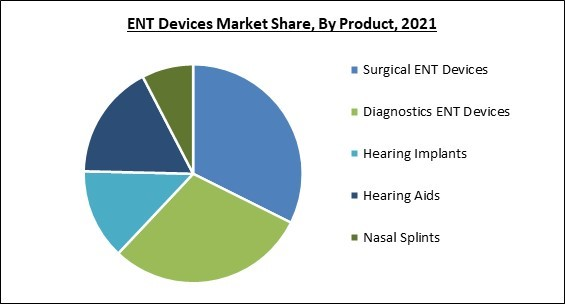
Based on product, the ENT Devices market is segmented into diagnostic ENT devices, surgical ENT devices, hearing aids, hearing implants and nasal splints. In 2021, the hearing aids segment procured a promising growth rate in the ENT Devices market. Most people who are 65 and older experience some degree of hearing loss due to aging. Many people do not become aware of this sort of hearing loss until it becomes a major issue since it occurs so gradually. Hearing aids cannot reverse hearing loss due to nerve damage or aging or stop the course of these conditions.End-Use Outlook
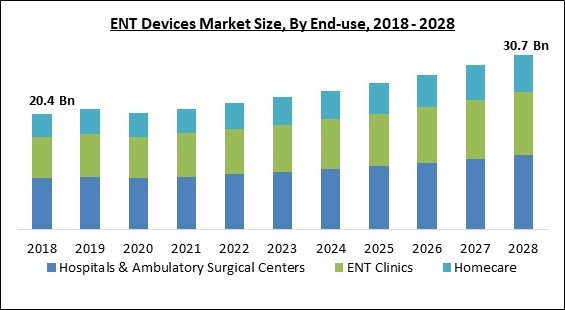
By end-use, the ENT device market is classified into hospitals & ambulatory surgical centers, ENT Clinics and homecare. In 2021, the hospitals & ambulatory surgical centers segment witnessed largest revenue share in the ENT device market. The market is expending in this segment due to the rising spending in healthcare industry to improve healthcare infrastructure. Furthermore, the ENT devices are in high demand in hospitals due to rising preference of people to visit hospitals directly and the quality care offered by these centers.Regional Outlook
Region wise, the ENT Devices market is analyzed across the North America, Europe, Asia Pacific and LAMEA. In 2021, the North America region led the ENT devices market by generating maximum revenue share. This is due to a number of factors, including the region's high prevalence of ENT-related illnesses, increasing preference for hearing care equipment, the presence of major hospital chains, well-equipped ambulatory facilities, and a sizable target market.The Cardinal Matrix - ENT Devices Market Competition Analysis
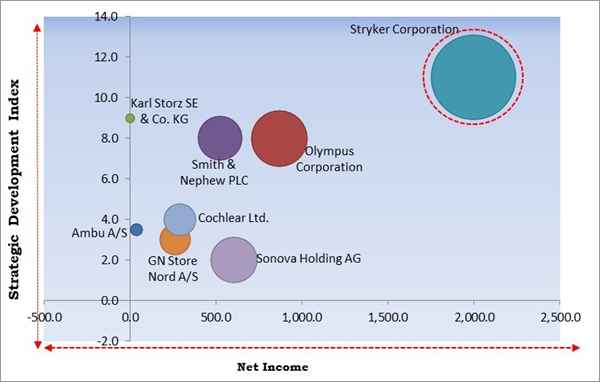
The major strategies followed by the market participants are Product Launches. Based on the Analysis presented in the Cardinal matrix; Stryker Corporation is the major forerunner in the ENT Devices Market. Companies such as Olympus Corporation, Sonova Holding AG and Smith & Nephew PLC are some of the key innovators in ENT Devices Market.
The market research report covers the analysis of key stakeholders of the market. Key companies profiled in the report include Smith & Nephew PLC, Sonova Holding AG, GN Store Nord A/S, Ambu A/S, Stryker Corporation, Karl Storz SE & Co. KG, Olympus Corporation, Richard Wolf GmbH, Cochlear, Ltd., and Starkey Laboratories, Inc.
Strategies Deployed in ENT Devices Market
Partnerships, Collaborations and Agreements:
- Aug-2022: Sonova Holding AG signed an agreement to acquire HYSOUND Group, a provider of hearing solutions company. This acquisition aims to strengthen Sonova’s direct consumer access and store footprint through the addition of 200 clinics in more than 20 provinces across 70 cities in China.
- Jul-2022: Smith & Nephew came into partnership with Rods&Cones, a provider of digital remote assistance platforms and smart surgery glasses. Together, the companies aimed to deliver smart surgery glasses and digital remote assistance to customers.
- May-2020: Smith & Nephew signed a distribution agreement with Fiagon, a provider of electromagnetic surgical navigation solutions company. Through this agreement, the former company aimed to distribute its ENT suite in the Asia Pacific region including Australia, India subcontinent, China, New Zealand, and ASEAN Markets.
Product Launches and Product Expansions:
- Sep-2022: Olympus unveiled VISERA ELITE III, a surgical visualization platform for endoscopic surgery. The product integrates the VISERA ELITE II system’s 3D, infrared imaging features and VISERA 4K UHD system’s 4K imaging feature. The VISERA ELITE III is created to serve the demands of healthcare professionals for endoscopic procedures in various medical specialties.
- Aug-2022: GN Hearing introduced ReSound OMNIA, a hearing aid platform that will set a new benchmark in hearing technology. This product is integrated with flawless connection to devices and optimal comfort to sound natural in the span of providing 150% improvement in speech understanding in contrast with current hearing aids.
- Mar-2022: Stryker released Power-PRO 2 powered ambulance cot, a connected ambulance cot created to deliver better safety, connectivity tools, and maneuverability to advance budget and time. This product is a reconceptualization of better representation and focuses on offering patients with foremost medical care.
- Jan-2022: Ambu unveiled aCart platform. The platform comprises of aCart Compact and aCart Plus for placement and transportation of endoscopes, displaying units, and additional accessories. The carts are developed to enhance operations over hospital sites of care consisting of Intensive Care Units, endoscope offerings, and operating rooms.
- Aug-2021: Karl Storz Endoscopy-America, Inc., a subsidiary of Karl Storz SE & Co. KG, is adding capabilities to TELE PACK + compact endoscopy system. The product now aligns with the data-management system and StreamConnect® networking. The TELE PACK+ compact endoscopy system is a movable video system that consists of various important components consisting a camera control unit, display, and light source used in the treatment and diagnosis of endoscopy.
- Jan-2021: Karl Storz Endoscopy-America, Inc., a subsidiary of Karl Storz SE & Co. KG, announced the launch of the IMAGE1 S Rubina multimode visualization system. The Product integrates enhanced fluorescence-guided imaging using near-infrared light, 4K resolution, and indocyanine green dye (NIR/ICG). This launch provides its customers the capability to develop and enhance technologically through including ability as per the demand of patient care.
- Jan-2020: Karl Storz announced the release of VITOM 3D, a 3D Surgical Imaging System. The product delivers 3D imaging with changing magnification for the imaging of microsurgical and open interventions in multiple surgical areas. The VITOM 3D system complements the Karl Storz IMAGE1 S camera resulting in adaptable and firm endoscopy consisting of 3D and 4K technologies on a single platform.
Mergers & Acquisitions:
- Jan-2020: Smith & Nephew PLC completed the acquisition of Tusker Medical, Inc., a provider of technology to ENT surgeons. Through this acquisition, ENT offerings of Tula align with Smith & Nephew and enhance treatment choices for patients and surgeons.
Trails & Approvals:
- Feb-2022: Olympus received FDA approval for CELERIS single-use sinus debrider system. The product consists of a single-use sinus debrider for coagulation, removal of thin bone and soft tissue, cutting, and debriding in general sinus/rhinology and ENT procedures. Moreover, the CELERIS single-use sinus debrider system is created to maintain costs and reprocessing generally connected with a full debrider system.
- Jan-2022: Cochlear Limited received FDA approval for Cochlear Nucleus Implants. The product is used for single-sided deafness and unilateral hearing loss. Cochlear Nucleus Implants solution restores the approach to sound to match the lifestyle needs and better standard of life. Patients with unilateral hearing loss and single-sided deafness can look for cochlear implant treatment to listen from both ears.
Geographical Expansions:
- Oct-2022: Ambu expanded its geographical footprint by opening a new manufacturing facility in Ciudad Juárez, Mexico. The plant has a comprehensive capacity of around 323,000 square feet and ramps up future production and supply of products. Moreover, Ambu will enhance its reach and accessibility in North America to serve its customers.
Scope of the Study
By End-use
- Hospitals & Ambulatory Surgical Centers
- ENT Clinics
- Homecare
By Product
- Surgical ENT Devices
- ENT Hand Instruments
- Sinus Dilation Devices
- Otological Drill Burrs
- Radiofrequency Handpieces
- Others
- Diagnostics ENT Devices
- Rigid Endoscopes
- Sinuscopes
- Otoscopes
- Others
- Flexible Endoscopes
- Bronchoscopes
- Laryngoscopes
- Nasopharyngoscopes
- Robot Assisted Endoscope
- Hearing Screening Device
- Rigid Endoscopes
- Hearing Implants
- Hearing Aids
- Nasal Splints
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Smith & Nephew PLC
- Sonova Holding AG
- GN Store Nord A/S
- Ambu A/S
- Stryker Corporation
- Karl Storz SE & Co. KG
- Olympus Corporation
- Richard Wolf GmbH
- Cochlear, Ltd.
- Starkey Laboratories, Inc.
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Smith & Nephew PLC
- Sonova Holding AG
- GN Store Nord A/S
- Ambu A/S
- Stryker Corporation
- Karl Storz SE & Co. KG
- Olympus Corporation
- Richard Wolf GmbH
- Cochlear, Ltd.
- Starkey Laboratories, Inc.