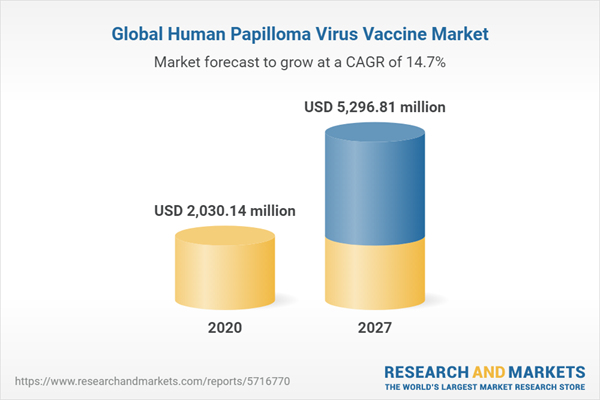
The human papilloma virus (HPV) market is projected to grow at a CAGR of 14.68% during the forecasted period to reach a market size of US$5,296.808 million by 2027, from US$2,030.136 million in 2020.
Along with other immunizations, routine vaccination is advised for all nations according to WHO guidelines. The Human Papilloma Virus (HPV) vaccine offers defense against specific strains of the virus that can cause genital warts or cancer. Along with other immunizations, routine vaccination is advised for all nations according to WHO guidelines. These vaccinations are regarded as safe and effective medications and are included on the World Health Organization's list of Essential Medicines. The global market for the Human Papilloma Virus (HPV) vaccine is being boosted by the rise in cervical cancer cases. Increased government measures will increase public awareness of HPV and cervical cancer, which will help the market for HPV vaccines. However, a stringent regulatory licensing procedure for the vaccines may limit market expansion.Effectiveness of HPV Vaccines to Boost Sales
The effectiveness and protection offered by HPV vaccines to lessen the negative effects associated with such infections are one of the most important and significant market drivers. Clinical trials have demonstrated the effectiveness and safety of both bivalent and polyvalent vaccinations in preventing HPV infections and cancer. Additionally, it is projected that the rising incidence of cervical cancer in various nations would boost sales of the human papillomavirus vaccine, resulting in a stellar market expansion. The market is also being driven by an increase in HPV vaccination production capacity to keep global supply and demand in balance.By Geography
Geographically, the Human Papilloma Virus Vaccine Market is segmented as North America, South America, Europe, the Middle East and Africa, and Asia Pacific. North America holds major share of the market whereas Asia-Pacific is anticipated to have a faster-growing market.Key Developments:
- June 2020: The FDA has authorized an additional indication for GARDASIL9 for the prevention of oropharyngeal and other head and neck cancers brought on by HPV Types 16, 18, 31, 33, 45, 52, and 58, according to a statement from Merck, often known as MSD. Based on its effectiveness in preventing HPV-related anogenital disease, the accelerated approval process has authorized the indication for oropharyngeal and head and neck cancer. The confirmation and description of clinical benefit in a confirmatory trial may be necessary in order for this indication to continue receiving approval. A vaccination called GARDASIL 9 is recommended for females between the ages of 9 and 45 for the prevention of genital warts, cervical, vulvar, vaginal, anal, oropharyngeal, and other head and neck malignancies brought on by the Human Papillomavirus (HPV). For the prevention of genital warts brought on by HPV and precancerous or dysplastic lesions as well as other head and neck cancers in males up to the age of 45, GARDASIL 9 is recommended.
- Dec 2021: Updates on the Phase 3 program for VGX-3100 for HPV-associated cervical high-grade squamous intraepithelial lesions (HSIL) have been made by INOVIO, a biotechnology firm focused on bringing precisely tailored DNA medicines to market to help protect people from infectious diseases and cure cancer and HPV-associated disorders. These updates include a one-year follow-up of efficacy and safety data in participants from REVEAL1, the completion of REVEAL2 enrolment, and In addition, ApolloBio Corp. , INOVIO's research partner in Greater China (mainland China, Hong Kong, Macao, Taiwan), dosed the first subject in a separate Phase 3 trial in China.
Product Offerings:
- GARDASIL 9 vaccine: GARDASIL 9 vaccine by Merck is a vaccination that is recommended for females between the ages of 9 and 45 for Human Papillomavirus (HPV) Types 6, 11, 16, 18, 31, 33, 45, 52, and 58 causing cervical, vulvar, vaginal, anal, oropharyngeal, and other head and neck cancers and Types 6, 11, and 6 and 11 of the HPV are also responsible for genital warts. GARDASIL 9 is recommended for use in males between the ages of 9 and 45 in order to prevent genital warts brought on by HPV Types 6 and 11, as well as anal, oropharyngeal, and other head and neck malignancies brought on by HPV Types 6, 11, 16, 18, 31, 33, 45, 52, and 58. Based on its effectiveness in preventing HPV-related anogenital disease, the accelerated approval process has authorized the indication for oropharyngeal and head and neck cancer.
- Cervarix vaccine: The GlaxoSmithKline-produced vaccine Cervarix is recommended for use starting at age 9 in order to prevent premalignant anogenital lesions (cervical, vulvar, vaginal, and anal cancers), as well as cervical and anal cancers that are connected to specific oncogenic Human Papillomavirus (HPV) types ( HPV 16 and 18). Cervarix is used for deltoid intramuscular injection. It has been demonstrated that the Cervarix vaccine shields women from a significant portion of the precursor lesions of cervical cancer brought on by these two HPV strains.
Market Segmentation:
By Type
- Quadrivalent HPV Vaccine
- Bivalent HPV Vaccine
- 9-Valent HPV Vaccine
By Indication
- Cervical Cancer
- Anal Cancer
- Vaginal Cancer
- Vulvar Cancer
- Mouth Cancer
- Genital Warts
By Gender
- Male
- Female
By Geography
- North America
- USA
- Mexico
- Canada
- South America
- Brazil
- Argentina
- Others
- Europe
- Germany
- France
- United Kingdom
- Italy
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- Israel
- Others
- Asia Pacific
- India
- China
- Japan
- South Korea
- Taiwan
- Thailand
- Indonesia
- Others
Table of Contents
INTRODUCTION1.1. Market Overview
1.2. COVID-19 Scenario
1.3. Market Definition
1.4. Market Segmentation
RESEARCH METHODOLOGY
2.1. Research Data
2.2. Assumptions
EXECUTIVE SUMMARY
3.1. Research Highlights
MARKET DYNAMICS
4.1. Market Drivers
4.2. Market Restraints
4.3. Porter’s Five Forces Analysis
4.3.1. Bargaining Power of Suppliers
4.3.2. Bargaining Power of Buyers
4.3.3. Threat of New Entrants
4.3.4. Threat of Substitutes
4.3.5. Competitive Rivalry in the Industry
4.4. Industry Value Chain Analysis
HUMAN PAPILLOMA VIRUS VACCINE MARKET ANALYSIS, BY TYPE
5.1. Introduction
5.2. Quadrivalent HPV Vaccine
5.3. Bivalent HPV vaccine
5.4. 9-Valent HPV Vaccine
HUMAN PAPILLOMA VIRUS VACCINE MARKET ANALYSIS, BY INDICATION
6.1. Introduction
6.2. Cervical Cancer
6.3. Anal Cancer
6.4. Vaginal Cancer
6.5. Vulvar Cancer
6.6. Genital Cancer
6.7. Others
HUMAN PAPILLOMA VIRUS VACCINE MARKET ANALYSIS, BY GENDER
7.1. Introduction
7.2. Male
7.3. Female
HUMAN PAPILLOMA VIRUS VACCINE MARKET ANALYSIS, BY GEOGRAPHY
8.1. Introduction
8.2. North America
8.2.1. USA
8.2.2. Canada
8.2.3. Mexico
8.3. South America
8.3.1. Brazil
8.3.2. Argentina
8.3.3. Others
8.4. Europe
8.4.1. Germany
8.4.2. France
8.4.3. United Kingdom
8.4.4. Italy
8.4.5. Others
8.5. Middle East and Africa
8.5.1. Saudi Arabia
8.5.2. UAE
8.5.3. Israel
8.5.4. Others
8.6. Asia Pacific
8.6.1. China
8.6.2. Japan
8.6.3. India
8.6.4. South Korea
8.6.5. Taiwan
8.6.6. Thailand
8.6.7. Indonesia
8.6.8. Others
COMPETITIVE INTELLIGENCE
9.1. Major Players and Strategy Analysis
9.2. Emerging Players and Market Lucrativeness
9.3. Mergers, Acquisitions, Agreements, and Collaborations
9.4. Vendor Competitiveness Matrix
COMPANY PROFILES
10.1. Merck & Co., Inc.
10.2. GSK plc
10.3. INOVIO Pharmaceuticals
*List is not exhaustive
Companies Mentioned
- Merck & Co Inc.
- GSK plc
- INOVIO Pharmaceuticals Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 132 |
| Published | January 2023 |
| Forecast Period | 2020 - 2027 |
| Estimated Market Value ( USD | $ 2030.14 million |
| Forecasted Market Value ( USD | $ 5296.81 million |
| Compound Annual Growth Rate | 14.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 3 |