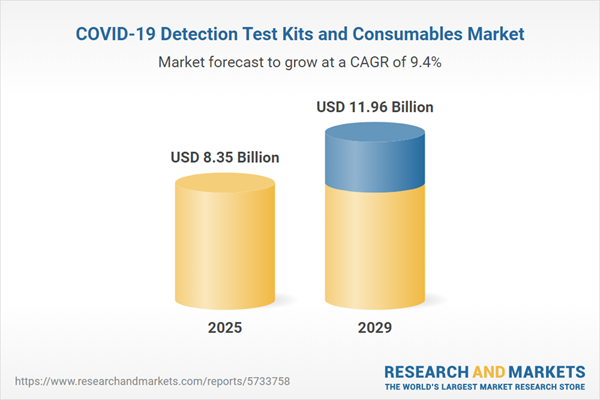
The COVID-19 detection test kits and consumables market size has grown strongly in recent years. It will grow from $7.79 billion in 2024 to $8.35 billion in 2025 at a compound annual growth rate (CAGR) of 7.2%. The growth in the historic period can be attributed to high prevalence of COVID-19, rising demand for early detection and diagnosis, increasing government support, growing awareness of the importance of testing, expanding reimbursement coverage.
The COVID-19 detection test kits and consumables market size is expected to see strong growth in the next few years. It will grow to $11.96 billion in 2029 at a compound annual growth rate (CAGR) of 9.4%. The growth in the forecast period can be attributed to growing demand for point-of-care testing, ongoing need for testing, emerging variants, vaccination campaigns, international travel and border controls. Major trends in the forecast period include multiplex testing, home testing kits, digital health integration, antibody tests for immunity.
The surge in demand for cost-effective and rapid COVID-19 detection kits, along with streamlined regulatory processes, has significantly bolstered the COVID-19 detection kits and consumables market. The global outbreak of COVID-19, designated a Public Health Emergency of International Concern (PHEIC) by the WHO, prompted a push for point-of-care diagnostics like Rapid Diagnostic Tests (RTDs). These tests, endorsed by the WHO, facilitate quick, on-site testing by minimally trained staff, delivering results within 15 minutes. Regulatory bodies like the US FDA responded by expediting approvals, allowing swift market introduction of COVID-19 detection kits. Consequently, the escalating global cases and the imperative for rapid diagnosis fuel the escalating demand for these efficient and accessible kits, steering the market's growth.
The rise in government initiatives is projected to drive the growth of the COVID-19 detection test kits and consumables market in the future. Government initiatives encompass actions and programs implemented by authorities to tackle specific challenges, achieve certain goals, or enhance the well-being of the population. These initiatives play a crucial role in fostering an environment conducive to the effective use of COVID-19 detection test kits and consumables, aiding the global efforts to manage and mitigate the pandemic's impact. For example, a report from the Centers for Medicare & Medicaid Services in December 2023 revealed that U.S. healthcare spending increased by 4.1% to reach $4.5 trillion in 2022, outpacing the 3.2% growth seen in 2021. As a result, the rise in government initiatives is propelling the growth of the COVID-19 detection test kits and consumables market.
CRISPR technology has revolutionized COVID-19 diagnostics, with the emergence of rapid diagnostic tests running on nasal swabs. Sherlock Biosciences received emergency use authorization (EUA) from the US FDA for its CRISPR-based test, marking a breakthrough as the first FDA-authorized CRISPR technology in the market. Mammoth Biosciences is also actively involved in the development of this innovative diagnostic technology.
Leading companies in the COVID-19 detection test kits and consumables market are increasingly pursuing strategic partnerships to deliver essential communication services to individuals, businesses, and governments. Strategic partnerships involve companies utilizing each other’s strengths and resources to achieve shared benefits and success. For example, in May 2022, Cipla Limited, a pharmaceutical company based in India, collaborated with Genes2Me Pvt. Ltd. to introduce the 'RT-Direct' multiplex COVID-19 RT-PCR test kit. This innovative diagnostic tool employs a unique RNA extraction-free process, significantly cutting the testing time down to just 45 minutes. The kit targets three specific genes associated with SARS-CoV-2, improving the test's sensitivity. With this launch, Cipla aims to enhance its diagnostics portfolio and facilitate COVID-19 testing across India.
Major companies operating in the COVID-19 detection test kits and consumables market include Cepheid, BGI Genomics, Abbott Laboratories, bioMérieux SA, Bio-Rad Laboratories, F. Hoffmann-La Roche, GenMark Diagnostics, Mylab Discovery Solutions, Qiagen, Quidel Corporation, Randox Laboratories, SD Biosensor, Seegene Inc., Shenzhen Bioeasy Biotechnology, Thermo Fisher Scientific inc., BioMednomics, Getein Biotech, Sensing Self Ltd., Hangzhou Biotest Biotech Co. Ltd, AmonMed Biotechnology Co., Beijing Tigsun Diagnostics Co Ltd., BioMaxima S.A., CTK Biotech, Hunan Lituo Biotechnology Co., Vivacheck Lab, MD Solutions, FastSense Diagnostics, Altona Diagnostics, Siemens AG, Agilent Technologies Inc., Seasun Biomaterials, BTNX Inc., Rapiim, Canon Medical Systems Corporation, Cellspect Co. Ltd., iHealth Labs Inc., InBios International Inc., iXensor Co. Ltd., Jiangsu Medomics medical technology Co Ltd., Maxim Biomedical Inc., Mologic Inc., OraSure Technologies Inc., OSANG LLC, PHASE Scientific International Ltd., Watmind USA, Xiamen Boson Biotech Co. Ltd.
COVID-19 detection test kits and consumables serve the purpose of identifying the novel COVID-19 (SARS-CoV-2) and utilize a laboratory technique known as reverse transcription polymerase chain reaction (RT-PCR) to detect the virus's genetic material.
The market for COVID-19 detection test kits and consumables is segmented into various categories. Kits include viral load testing kits such as qPCR and RT-PCR, virus-neutralizing assay kits, antibody detection kits (ELISA), viral antigen detection test kits, and other variants. Consumables comprise items like swabs, tubes, viral transfer media, reagents, and other consumables. Additionally, specimen types for testing encompass nose and throat swabs, blood samples, sputum, nasal aspirate, and similar materials. These test kits and consumables cater to diverse end-users, including hospitals, clinics, public health laboratories, private and commercial labs, physician labs, research institutes, and other entities involved in the diagnosis and monitoring of COVID-19 infections.
The COVID-19 detection test kits and consumables market research report is one of a series of new reports that provides COVID-19 detection test kits and consumables market statistics, including COVID-19 detection test kits and consumables industry global market size, regional shares, competitors with a COVID-19 detection test kits and consumables market share, detailed COVID-19 detection test kits and consumables market segments, COVID-19 detection test kits and consumables market trends and opportunities, and any further data you may need to thrive in the COVID-19 detection test kits and consumables industry. This COVID-19 detection test kits and consumables market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
North America was the largest region in the COVID-19 detection test kits and consumables market in 2024. The regions covered in the COVID-19 detection test kits and consumables market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, and Africa. The countries covered in the COVID-19 detection test kits and consumables market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
The COVID-19 detection test kits & consumables market consists of sales of nucleic acid amplification tests (NAATs) and antigen tests. Values in this market are factory gate values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
COVID-19 Detection Test Kits and Consumables Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on COVID-19 detection test kits and consumables market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for COVID-19 detection test kits and consumables ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The COVID-19 detection test kits and consumables market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Kits: Viral Load Testing Kits (qPCR and RT-PCR); Virus Neutralizing Assay Kits; Antibody Detection Kits (Elisa); Viral Antigen Detection Test Kits; Other Kits2) By Consumables: Swabs; Tubes; Viral Transfer Media; Reagents; Other Consumables
3) By Specimen Type: Nose & Throat Swab; Blood; Sputum; Nasal Aspirate
4) By End Use: Hospitals; Clinics; Public Health Labs; Private and Commercial Labs; Physicians Labs; Research Institutes; Other End Uses
Subsegments:
1) By Viral Load Testing Kits (QPCR and RT-PCR): QPCR Kits; RT-PCR Kits2) By Virus Neutralizing Assay Kits: Neutralization Test Kits
3) By Antibody Detection Kits (Elisa): IgG Detection Kits; IgM Detection Kits
4) By Viral Antigen Detection Test Kits: Rapid Antigen Test Kits
5) By Other Kits: Combination Test Kits; Multiplex Test Kits
Key Companies Mentioned: Cepheid; BGI Genomics; Abbott Laboratories; bioMérieux SA; Bio-Rad Laboratories
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this COVID-19 Detection Test Kits and Consumables market report include:- Cepheid
- BGI Genomics
- Abbott Laboratories
- bioMérieux SA
- Bio-Rad Laboratories
- F. Hoffmann-La Roche
- GenMark Diagnostics
- Mylab Discovery Solutions
- Qiagen
- Quidel Corporation
- Randox Laboratories
- SD Biosensor
- Seegene Inc.
- Shenzhen Bioeasy Biotechnology
- Thermo Fisher Scientific inc.
- BioMednomics
- Getein Biotech
- Sensing Self Ltd.
- Hangzhou Biotest Biotech Co. Ltd
- AmonMed Biotechnology Co.
- Beijing Tigsun Diagnostics Co Ltd.
- BioMaxima S.A.
- CTK Biotech
- Hunan Lituo Biotechnology Co.
- Vivacheck Lab
- MD Solutions
- FastSense Diagnostics
- Altona Diagnostics
- Siemens AG
- Agilent Technologies Inc.
- Seasun Biomaterials
- BTNX Inc.
- Rapiim
- Canon Medical Systems Corporation
- Cellspect Co. Ltd.
- iHealth Labs Inc.
- InBios International Inc.
- iXensor Co. Ltd.
- Jiangsu Medomics medical technology Co Ltd.
- Maxim Biomedical Inc.
- Mologic Inc.
- OraSure Technologies Inc.
- OSANG LLC
- PHASE Scientific International Ltd.
- Watmind USA
- Xiamen Boson Biotech Co. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 8.35 Billion |
| Forecasted Market Value ( USD | $ 11.96 Billion |
| Compound Annual Growth Rate | 9.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 46 |