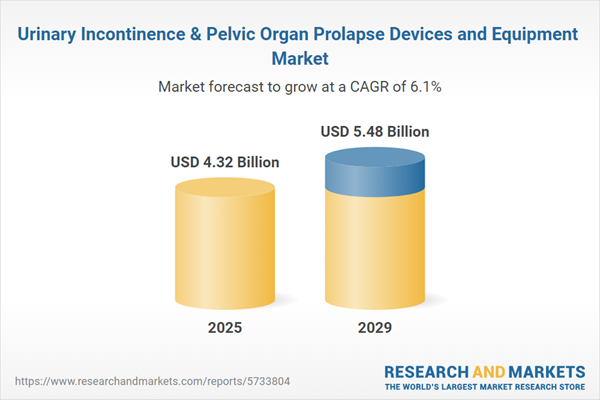
The urinary incontinence & pelvic organ prolapse devices and equipment market size has grown strongly in recent years. It will grow from $4.01 billion in 2024 to $4.32 billion in 2025 at a compound annual growth rate (CAGR) of 7.6%. The growth in the historic period can be attributed to aging population, women's health awareness, healthcare access, personalized treatment.
The urinary incontinence & pelvic organ prolapse devices and equipment market size is expected to see strong growth in the next few years. It will grow to $5.48 billion in 2029 at a compound annual growth rate (CAGR) of 6.1%. The growth in the forecast period can be attributed to minimally invasive techniques, emerging markets growth, value-based healthcare, regulatory support. Major trends in the forecast period include non-surgical treatment options, bioresorbable implants, digital health integration, enhanced patient education.
The increased prevalence of Pelvic Organ Prolapse (POP) and Urinary Incontinence (UI) disorders has been a driving force behind the growth of the market for urinary incontinence and pelvic organ prolapse (POP) devices and equipment. This rising prevalence has prompted manufacturers to develop more precise and effective devices for treating these conditions. While POP can affect women of all ages, it is more common in older women. According to reports from the US FDA, the number of women with POP is projected to increase by 46%, reaching 4.9 million by 2050. Additionally, a report from the American Urological Association (AUA/SUFU) found that the prevalence of stress urinary incontinence (SUI) in women can be as high as 49%. Poor nutritional status can lead to a higher prevalence of SUI due to the weakening of pelvic support. This increased prevalence is fueling the demand for medical equipment in the urinary incontinence and pelvic organ prolapse devices and equipment market.
The increasing geriatric population is expected to drive the growth of the urinary incontinence and pelvic organ prolapse devices and equipment market. The geriatric population refers to individuals aged 65 and older. Urinary incontinence and pelvic organ prolapse are prevalent health issues among the elderly, and various devices and equipment are utilized to manage these conditions. For example, in October 2023, HelpAge International, a non-governmental organization advocating for the rights of older people, projected that by 2050, 80% of the world's elderly population will live in low- and middle-income countries. This growing elderly demographic is fueling demand in markets such as urinary incontinence and pelvic organ prolapse devices.
Prominent companies in the urinary incontinence and pelvic organ prolapse devices and equipment market are introducing innovative solutions like the SOLTIVE Super Pulsed Laser System to enhance their market revenue. The SOLTIVE Premium Super Pulsed Laser System, known as the SOLTIVE Laser System, has the potential to reduce procedure duration, improve patient outcomes, and lower the overall procedure costs. This system is applied in the treatment of urinary incontinence and various urological conditions, including the removal of kidney stones. For instance, in January 2022, Olympus, a Japanese company specializing in digital precision technology, introduced the SOLTIVE Super Pulsed Laser System, which offers significant advantages in terms of shorter procedure times and cost-effectiveness when compared to Holmium. The SOLTIVE Laser System, on average, reduces procedure times by 20% and holds the promise of achieving better patient outcomes.
Devices such as the Lyrette transurethral SUI system are employed in procedures to address incontinence. Lyrette treatment does not necessitate the use of anesthesia or surgery; instead, it utilizes radiofrequency energy to tighten the bladder tissue. In the case of treating pelvic organ prolapse (POP), companies are shifting their focus towards native tissue prolapse repair procedures rather than relying on vaginal mesh, following the emergence of health concerns associated with its use.
Regulatory bodies, including the FDA in the United States, play a pivotal role in overseeing the manufacturing and safety standards for devices and equipment related to urinary incontinence and pelvic organ prolapse disorders. For instance, manufacturers are mandated to obtain pre-market clearance (510(k)) for tools specifically designed for implanting surgical mesh. The FDA further requires manufacturers in the urinary incontinence and pelvic organ prolapse devices and equipment industry to conduct post-market studies to assess the safety and effectiveness of surgical mesh used in POP repair. As a result, FDA regulations have led to the removal of certain products from the market and compelled companies like Coloplast and Acell to discontinue marketing their respective products, namely Coloplast's Restorelle DirectFix Posterior and Acell Matristem's Pelvic Floor Repair Matrix.
Urinary incontinence & pelvic organ prolapse devices and equipment are used in the treatment of pelvic organ prolapse and urinary incontinence. Urinary incontinence (UI), also known as involuntary urination, is defined as uncontrolled leakage of urine caused by an overactive bladder. Pelvic organ prolapse (POP) is caused by defects in the supporting structures of the vagina.
These devices can be categorized into two main types such as urinary incontinence devices and pelvic organ prolapse devices. Urinary incontinence devices encompass items like artificial urinary sphincters, electrical stimulation devices, urethral slings, and catheters, which are utilized to address urinary incontinence. In contrast, pelvic organ prolapse devices include vaginal mesh and vaginal pessaries, designed to manage pelvic organ prolapse. Urinary incontinence can manifest in various forms, including stress incontinence, urge incontinence, overflow incontinence, and functional incontinence. These devices find applications across different healthcare settings, such as hospitals, clinics, ambulatory surgical centers, and for at-home use.
The urinary incontinence & pelvic organ prolapse devices and equipment market research report is one of a series of new reports that provides urinary incontinence & pelvic organ prolapse devices and equipment market statistics, including urinary incontinence & pelvic organ prolapse devices and equipment industry global market size, regional shares, competitors with a urinary incontinence & pelvic organ prolapse devices and equipment market share, detailed urinary incontinence & pelvic organ prolapse devices and equipment market segments, market trends and opportunities, and any further data you may need to thrive in the urinary incontinence & pelvic organ prolapse devices and equipment industry. This urinary incontinence & pelvic organ prolapse devices and equipment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Major companies operating in the urinary incontinence & pelvic organ prolapse devices and equipment market include Boston Scientific Corporation, Coloplast A/S, Ethicon US, Becton Dickinson and Company, Johnson & Johnson, Teleflex Incorporated, Medtronic plc, COVIDien, Cook Medical, Neomedic International, CooperSurgical, MedGyn, Personal Medical Corp, Integra LifeSciences, Panpac Medical, Medesign, Smiths Medical Inc., Thomas Medical, Kangge Medical, Dr. Arabin GmbH & Co. KG, American Medical Systems Inc., Caldera Medical Inc., Intuitive Surgical, InControl Medical LLC, Atlantic Therapeutics Ltd., Axonics Modulation Technologies, Convatec Group plc, Laborie, NUVO Group, Pelvalon, pfm medical, Promedon, Prosurg Inc., UROMED Kurt Drews KG, Verathon Inc., Vitality Medical, Women's Choice Pharmaceuticals, Zimmer Biomet Holdings Inc.
North America was the largest region in the urinary incontinence & pelvic organ prolapse devices and equipment market in 2024. Western Europe was the second-largest region in the urinary incontinence & pelvic organ prolapse devices and equipment market report. The regions covered in the urinary incontinence & pelvic organ prolapse devices and equipment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, and Africa. The countries covered in the urinary incontinence & pelvic organ prolapse devices and equipment market report are Australia, China, India, Indonesia, Japan, South Korea, Bangladesh, Thailand, Vietnam, Malaysia, Singapore, Philippines, Hong Kong, New Zealand, USA, Canada, Mexico, Brazil, Chile, Argentina, Colombia, Peru, France, Germany, UK, Austria, Belgium, Denmark, Finland, Ireland, Italy, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, Russia, Czech Republic, Poland, Romania, Ukraine, Saudi Arabia, Israel, Iran, Turkey, UAE, Egypt, Nigeria, South Africa.
The urinary incontinence & pelvic organ prolapse devices and equipment market consist of sales of artificial urinary sphincters, electrical stimulation devices, urethral slings, and catheters that are used for the treatment of pelvic organ prolapse and urinary incontinence. Values in this market are factory gate values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Executive Summary
Urinary Incontinence & Pelvic Organ Prolapse Devices and Equipment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on urinary incontinence & pelvic organ prolapse devices and equipment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 50 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for urinary incontinence & pelvic organ prolapse devices and equipment ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The urinary incontinence & pelvic organ prolapse devices and equipment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Urinary Incontinence Devices; Pelvic Organ Prolapse Devices2) By Urinary Incontinence Devices: Artificial Urinary Sphincters; Electrical Stimulation Devices; Urethral Slings; Catheters
3) By Pelvic Organ Prolapse Devices: Vaginal Mesh; Vaginal Pessary
4) By Incontinence Type: Stress Incontinence; Urge Incontinence; Overflow Incontinence; Functional Incontinence
5) By End User: Hospitals; Clinics; Ambulatory Surgical Centers; Home Use
Subsegments:
1) By Urinary Incontinence Devices: Catheters (Intermittent, Indwelling, External); Absorbent Products (Pads, Adult Diapers); External Urinary Collection Devices; Electrical Stimulation Devices; Urethral Inserts; Artificial Urinary Sphincter2) By Pelvic Organ Prolapse Devices: Vaginal Pessaries; Pelvic Floor Stimulation Devices; Surgical Implants; Prolapse Support Devices
Key Companies Mentioned: Boston Scientific Corporation; Coloplast a/S; Ethicon US; Becton Dickinson and Company; Johnson & Johnson
Countries: Australia; China; India; Indonesia; Japan; South Korea; Bangladesh; Thailand; Vietnam; Malaysia; Singapore; Philippines; Hong Kong; New Zealand; USA; Canada; Mexico; Brazil; Chile; Argentina; Colombia; Peru; France; Germany; UK; Austria; Belgium; Denmark; Finland; Ireland; Italy; Netherlands; Norway; Portugal; Spain; Sweden; Switzerland; Russia; Czech Republic; Poland; Romania; Ukraine; Saudi Arabia; Israel; Iran; Turkey; UAE; Egypt; Nigeria; South Africa
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Urinary Incontinence & Pelvic Organ Prolapse Devices and Equipment market report include:- Boston Scientific Corporation
- Coloplast A/S
- Ethicon US
- Becton Dickinson and Company
- Johnson & Johnson
- Teleflex Incorporated
- Medtronic plc
- COVIDien
- Cook Medical
- Neomedic International
- CooperSurgical
- MedGyn
- Personal Medical Corp
- Integra LifeSciences
- Panpac Medical
- Medesign
- Smiths Medical Inc.
- Thomas Medical
- Kangge Medical
- Dr. Arabin GmbH & Co. KG
- American Medical Systems Inc.
- Caldera Medical Inc.
- Intuitive Surgical
- InControl Medical LLC
- Atlantic Therapeutics Ltd.
- Axonics Modulation Technologies
- Convatec Group plc
- Laborie
- NUVO Group
- Pelvalon
- pfm medical
- Promedon
- Prosurg Inc.
- UROMED Kurt Drews KG
- Verathon Inc.
- Vitality Medical
- Women's Choice Pharmaceuticals
- Zimmer Biomet Holdings Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 4.32 Billion |
| Forecasted Market Value ( USD | $ 5.48 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 38 |