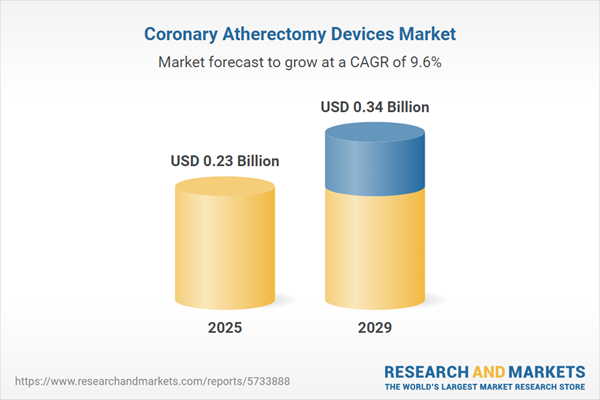
The coronary atherectomy devices market size has grown strongly in recent years. It will grow from $0.21 billion in 2024 to $0.23 billion in 2025 at a compound annual growth rate (CAGR) of 9.5%. The growth in the historic period can be attributed to rising prevalence of coronary artery disease, growing demand for minimally invasive procedures, demand for coronary atherectomy devices, rising disposable incomes.
The coronary atherectomy devices market size is expected to see strong growth in the next few years. It will grow to $0.34 billion in 2029 at a compound annual growth rate (CAGR) of 9.6%. The growth in the forecast period can be attributed to aging population, hybrid procedures, industry investment, patient and physician awareness. Major trends in the forecast period include advancements in coronary atherectomy devices, miniaturization, enhanced imaging, personalized treatment, advancements in device design, robotics and artificial intelligence.
The growing incidence of cardiovascular diseases (CVDs) has led to a heightened demand for atherectomy devices in the treatment of CVDs. This surge in CVD cases can be attributed to factors such as smoking, physical inactivity, and unhealthy dietary habits. As of September 2023, the Center for Disease Control and Prevention reported that the United States recorded 695,547 deaths attributed to cardiovascular diseases in 2021. Furthermore, the American College of Cardiology, in its August 2022 findings, predicted a 30.7% increase in risk factors for ischemic heart disease, projecting a total of 29 million cases by 2025. Consequently, the escalating prevalence of cardiovascular diseases is anticipated to be a driving force behind the global coronary atherectomy devices market's growth.
Increasing healthcare spending is poised to fuel the expansion of the coronary atherectomy devices market. Healthcare expenditure constitutes the overall costs invested in healthcare-related products and services within a specific healthcare system or economy. This investment plays a vital role in fostering the development, accessibility, and utilization of coronary atherectomy devices and treatments. For instance, as per the November 2022 report by the Canadian Institute for Health Information, healthcare spending surged to $331 billion, a rise of 0.8% from the previous year's 7.6% in 2021. Hence, the upward trajectory of healthcare expenditure is a significant driver behind the growth of the coronary atherectomy devices market.
Leading companies in the coronary atherectomy equipment market are emphasizing innovative solutions, such as radial access catheters, to enhance patient outcomes by enabling safer and less invasive treatments for coronary artery disease. Radial access catheters are specialized devices used in interventional cardiology to access coronary arteries via the radial artery in the wrist, as opposed to the traditional femoral artery in the groin. For example, in January 2024, AngioDynamics, a US-based provider of healthcare products and solutions, received FDA 510(k) clearance for its Auryon XL Radial Access Catheter, developed for the treatment of peripheral arterial disease (PAD). This 225-cm catheter, compatible with the Auryon Atherectomy System, is available in two diameters (0.9 mm and 1.5 mm) and leverages advanced laser technology for effective arterial occlusion treatment. Radial access offers significant advantages, including a lower risk of major bleeding compared to femoral access and the ability to treat bilateral disease in a single session, facilitating quicker patient recovery.
Prominent companies in the coronary atherectomy devices market are intensifying their efforts to introduce cutting-edge devices like orbital atherectomy systems, aiming to secure a competitive advantage in the market. The orbital atherectomy system serves as a medical device dedicated to addressing calcified plaque in both coronary and peripheral arteries. Distinguished by its distinct mode of action, the system is employed for preparing calcified plaque lesions prior to percutaneous coronary intervention (PCI) and treating peripheral artery disease (PAD). As an example, in October 2022, Cardiovascular Systems, Inc., a US-based medical device company, unveiled the Peripheral Orbital Atherectomy Systems 2.0 Max Crown. This innovative device incorporates a larger 70-micron diamond-coated crown, enhancing its effectiveness in engaging mixed plaque and ensuring superior outcomes. Particularly for individuals with peripheral artery disease, the Diamondback 360 Peripheral Orbital Atherectomy System offers discreet minimally invasive treatment options.
In April 2023, Abbott Laboratories, a US-based medical device firm, finalized the acquisition of Cardiovascular Systems, Inc. for $890 million. Through this acquisition, Abbott aims to enhance their capacity to cater to patients dealing with peripheral and coronary artery disease, particularly by preparing the arteries for angioplasty or stenting procedures to restore adequate blood flow. Cardiovascular Systems, Inc., a US-based medical device company, specializes in developing advanced treatments for coronary artery disease and challenging peripheral vascular conditions.
Coronary atherectomy devices serve the purpose of removing atherosclerosis from coronary blood vessels. These devices are specifically engineered to eliminate atheroma, calcium, and excess cellular material from areas of coronary occlusion or stenosis.
The primary products in coronary atherectomy devices include directional atherectomy devices, orbital atherectomy devices, photo-ablative atherectomy devices, rotational atherectomy devices, among others. Rotational atherectomy devices, for instance, consist of a lengthy catheter housing a microscopic diamond-embedded, oval-shaped burr for the purpose of effectively addressing coronary conditions. These devices are widely utilized across various applications such as peripheral vascular, cardiovascular, and neurovascular treatments. They are employed by a range of end-users, including hospitals, surgical centers, ambulatory care facilities, research laboratories, and academic institutes.
The coronary atherectomy devices market research report is one of a series of new reports that provides coronary atherectomy devices market statistics, including coronary atherectomy devices industry global market size, regional shares, competitors with a coronary atherectomy devices market share, detailed coronary atherectomy devices market segments, market trends and opportunities, and any further data you may need to thrive in the coronary atherectomy devices industry. This coronary atherectomy devices market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Major companies operating in the coronary atherectomy devices market include Boston Scientific Corporation, Koninklijke Philips N.V., Cardiovascular Systems Inc., The Spectranetics Corporation, Medtronic Inc., Avinger Inc., Biotronik SE & Co KG, Arterial Remodeling Technologies, Atrium Medical Corporation, B. Braun Melsungen AG, Balton Sp. z o.o., Biosensors International Group Ltd., Straub Medical AG, C.R. Bard Inc., RA Medical Systems Inc., Shockwave Medical Inc., Terumo Corporation, Abbott Laboratories Inc., Asahi Intecc Co. Ltd., Baylis Medical Company Inc., Cook Medical LLC, Cordis Corporation, Edwards Lifesciences Corporation, Endologix Inc., Infraredx Inc., Intact Vascular Inc., Lutonix Inc., Mercator MedSystems Inc., Miracor Medical Systems GmbH, OrbusNeich Medical Company Limited, QT Vascular Ltd., TriReme Medical LLC, Vascular Solutions Inc., Volcano Corporation.
North America was the largest region in the coronary atherectomy devices market in 2024. Western Europe was the second-largest region in the global coronary atherectomy devices market share. Africa, was the smallest region in the coronary atherectomy devices industry. The regions covered in the coronary atherectomy devices market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, and Africa. The countries covered in the coronary atherectomy devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
The coronary atherectomy devices market consists of sales of directional atherectomy devices, orbital atherectomy devices, photo-ablative atherectomy devices, rotational atherectomy devices, and others that are used to treat coronary atherectomy. Values in this market are factory gate values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Executive Summary
Coronary Atherectomy Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on coronary atherectomy devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 50 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for coronary atherectomy devices ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The coronary atherectomy devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Directional Atherectomy Devices; Orbital Atherectomy Devices; Photo-Ablative Atherectomy Devices; Rotational Atherectomy Devices; Other Products2) By Application: Peripheral Vascular; Cardiovascular; Neurovascular
3) By End User: Hospitals and Surgical Centers; Ambulatory Care Centers; Research Laboratories and Academic Institutes
Subsegments:
1) By Directional Atherectomy Devices: Traditional Directional Atherectomy Devices; Advanced Directional Atherectomy Devices2) By Orbital Atherectomy Devices: Single-Wire Orbital Atherectomy Devices; Multi-Wire Orbital Atherectomy Devices
3) By Photo-Ablative Atherectomy Devices: Laser-Based Photo-Ablative Devices; Other Photo-Ablative Atherectomy Devices
4) By Rotational Atherectomy Devices: Manual Rotational Atherectomy Devices; Motorized Rotational Atherectomy Devices
5) By Other Products: Combination Atherectomy Devices; Custom Atherectomy Solutions; Other Coronary Atherectomy Devices
Key Companies Mentioned: Boston Scientific Corporation; Koninklijke Philips N.V.; Cardiovascular Systems Inc.; the Spectranetics Corporation; Medtronic Inc.
Countries: Australia; China; India; Indonesia; Japan; South Korea; Bangladesh; Thailand; Vietnam; Malaysia; Singapore; Philippines; Hong Kong; New Zealand; USA; Canada; Mexico; Brazil; Chile; Argentina; Colombia; Peru; France; Germany; UK; Austria; Belgium; Denmark; Finland; Ireland; Italy; Netherlands; Norway; Portugal; Spain; Sweden; Switzerland; Russia; Czech Republic; Poland; Romania; Ukraine; Saudi Arabia; Israel; Iran; Turkey; UAE; Egypt; Nigeria; South Africa
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Coronary Atherectomy Devices market report include:- Boston Scientific Corporation
- Koninklijke Philips N.V.
- Cardiovascular Systems Inc.
- The Spectranetics Corporation
- Medtronic Inc.
- Avinger Inc.
- Biotronik SE & Co KG
- Arterial Remodeling Technologies
- Atrium Medical Corporation
- B. Braun Melsungen AG
- Balton Sp. z o.o.
- Biosensors International Group Ltd.
- Straub Medical AG
- C.R. Bard Inc.
- RA Medical Systems Inc.
- Shockwave Medical Inc.
- Terumo Corporation
- Abbott Laboratories Inc.
- Asahi Intecc Co. Ltd.
- Baylis Medical Company Inc.
- Cook Medical LLC
- Cordis Corporation
- Edwards Lifesciences Corporation
- Endologix Inc.
- Infraredx Inc.
- Intact Vascular Inc.
- Lutonix Inc.
- Mercator MedSystems Inc.
- Miracor Medical Systems GmbH
- OrbusNeich Medical Company Limited
- QT Vascular Ltd.
- TriReme Medical LLC
- Vascular Solutions Inc.
- Volcano Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 0.23 Billion |
| Forecasted Market Value ( USD | $ 0.34 Billion |
| Compound Annual Growth Rate | 9.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 35 |