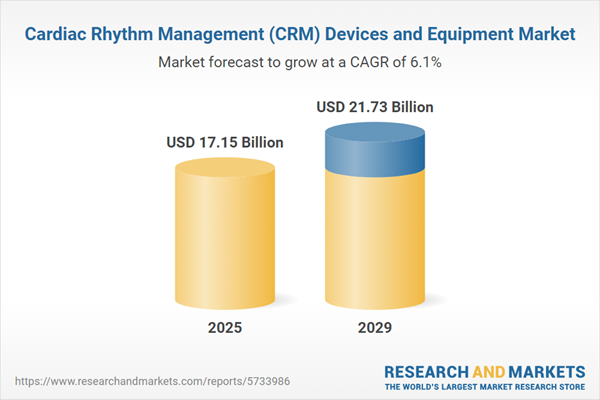
The cardiac rhythm management (CRM) devices and equipment market size has grown strongly in recent years. It will grow from $16.05 billion in 2024 to $17.15 billion in 2025 at a compound annual growth rate (CAGR) of 6.8%. The growth in the historic period can be attributed to prevalence of arrhythmias, technological advancements, aging population, chronic disease management.
The cardiac rhythm management (CRM) devices and equipment market size is expected to see strong growth in the next few years. It will grow to $21.73 billion in 2029 at a compound annual growth rate (CAGR) of 6.1%. The growth in the forecast period can be attributed to personalized medicine, technological innovations, telehealth integration, global access to healthcare. Major trends in the forecast period include leadless crm devices, biodegradable devices, remote monitoring, combination therapies.
The surge in the elderly demographic is anticipated to drive the growth of the cardiac rhythm management (CRM) devices and equipment market. As the population of individuals aged 65 and above continues to expand, the prevalence of cardiovascular ailments increases, resulting in a heightened demand for CRM devices. These tools are vital in addressing heart rhythm disorders like arrhythmias and significantly improving the quality of life for the elderly. According to the Administration for Community Living, the senior population reached 55.8 million in 2020, projected to reach 80.8 million by 2040 and 94.7 million by 2060. This demographic shift is a key driver behind the expanding CRM devices and equipment market.
The increasing prevalence of cardiovascular diseases is expected to drive the growth of the cardiac rhythm management (CRM) devices and equipment market in the coming years. Cardiovascular diseases encompass a range of disorders affecting the heart and blood vessels, including coronary artery disease, heart attacks, strokes, hypertension (high blood pressure), heart failure, and arrhythmias. The rising incidence of these conditions is largely attributed to unhealthy lifestyle choices, such as poor diet and lack of physical activity. CRM devices and equipment play a crucial role in diagnosing, monitoring, and treating cardiovascular diseases, particularly those involving abnormal heart rhythms (arrhythmias). For instance, a report published in September 2024 by the British Heart Foundation, a UK-based cardiovascular research charity, revealed that approximately 7.6 million people in the UK are affected by heart and circulatory diseases, including 4 million men and 3.6 million women. The report also highlighted that around 49,000 individuals under 75 die from these conditions annually. As such, the rising incidence of cardiovascular diseases is driving the growth of the CRM devices and equipment market.
Key players in the cardiac rhythm management (CRM) devices and equipment market are innovating by developing advanced products such as the single-chamber (VR) leadless pacemaker, aimed at managing heart rhythm issues and enhancing patient outcomes. This leadless pacemaker is directly implanted into the heart's right ventricle via a minimally invasive procedure, eliminating the need for cardiac leads. Abbott Laboratories, a US-based medical device company, received FDA approval in April 2022 for the Aveir single-chamber (VR) leadless pacemaker to address slow heart rhythms in patients. This device offers comprehensive insights into patient health and heart activity, enabling better treatment decisions and enhancing patient outcomes. The Aveir VR leadless pacemaker's software has been designed for potential expansion to a dual-chamber system, promising additional treatment options for future patients.
Innovative technologies like the subcutaneous implantable cardioverter-defibrillator (S-ICD) and leadless cardiac pacemakers (LCP) aim to address complications associated with transvenous lead and other cardiac rhythm management (CRM) devices. A modern modular cardiac rhythm management (mCRM) system facilitates wireless communication between devices for coordinated leadless pacing and defibrillator therapy. This system allows anti-tachycardia pacing-enabled LCP and S-ICD to communicate within the body. For example, Medtronic's Micra, a leadless pacemaker, is vein-inserted and self-contained within the heart.
Cardiac rhythm management device manufacturers are subject to regulation by authorities like the FDA in the United States. The FDA ensures product safety and addresses any flaws that could potentially jeopardize consumer well-being. For instance, a recall was initiated by the FDA on the Platinium implantable cardiac defibrillator and resynchronization therapy defibrillator due to concerns regarding hardware configuration and performance. The company was obliged to remove all non-implanted Platinium devices and follow up with patients who had these devices implanted.
Cardiac rhythm management (CRM) devices and equipment play a vital role in regulating heart rate and rhythm by administering electrical impulses or shocks to restore normal heart function. These devices are instrumental in managing arrhythmia-related conditions like cardiac arrests, heart failure, and various cardiac arrhythmias.
The primary products in the realm of cardiac rhythm management devices are pacemakers, defibrillators, and cardiac resynchronization therapy (CRT). Pacemakers, for instance, are compact devices implanted in the chest to regulate heartbeats, preventing slow heart rates. These devices serve purposes such as managing bradycardia, tachycardia, heart failure, and other related conditions. They are employed across different medical facilities, including hospitals, ambulatory surgical centers, and physician clinics.
The cardiac rhythm management (CRM) devices and equipment market research report is one of a series of new reports that provides cardiac rhythm management (CRM) devices and equipment market statistics, including cardiac rhythm management (CRM) devices and equipment industry global market size, regional shares, competitors with a cardiac rhythm management (CRM) devices and equipment market share, detailed cardiac rhythm management (CRM) devices and equipment market segments, market trends and opportunities, and any further data you may need to thrive in the cardiac rhythm management (CRM) devices and equipment industry. This cardiac rhythm management (CRM) devices and equipment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Major companies operating in the cardiac rhythm management (CRM) devices and equipment market include Abbott Laboratories, Medtronic PLC, Biotronik SE & Co KG, Cardiac Science Corporation, Schiller AG, Hill-Rom Holdings Inc., GE Healthcare, Integer Holdings, Neovasc Inc., Philips Healthcare, Stryker Corporation, Rochling Medical, CCC Medical Devices, LivaNova PLC, ZOLL Medical Corporation, Koninklijke Philips N.V, Applied Cardiac System, Cook Medical, Abiomed Inc., Berlin Heart GmbH, Brotionik SE and Co. KG, Jarvik Hearth Inc., MicroPort Scientific Corp, ReliantHeart Inc., Shree Pacetronix Ltd., Shaanxi Qinming Medical Instrument Co. Ltd., AtriCure Inc., Boston Scientific Corporation, Edwards Lifesciences Corporation, Progetti Srl.
North America was the largest region in the global cardiac rhythm management (CRM) devices and equipment market in 2024. Western Europe was the second-largest region in the cardiac rhythm management (CRM) devices and equipment market. The regions covered in the cardiac rhythm management (CRM) devices and equipment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, and Africa. The countries covered in the cardiac rhythm management (CRM) devices and equipment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
The cardiac rhythm management (CRM) devices and equipment market consist of sales of Pacemakers, (implantable pacemakers, external pacemakers), defibrillators (implantable cardioverter defibrillators, external defibrillators), and cardiac resynchronization therapy (CRT-defibrillators, CRT-pacemakers) that are used for rhythm management. Values in this market are factory gate values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Executive Summary
Cardiac Rhythm Management (CRM) Devices and Equipment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on cardiac rhythm management (crm) devices and equipment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 50 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for cardiac rhythm management (crm) devices and equipment ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The cardiac rhythm management (crm) devices and equipment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Pacemakers; Defibrillators; Cardiac Resynchronization Therapy (CRT)2) By Application: Bradycardia; Tachycardia; Heart Failure; Other Applications
3) By End User: Hospitals; Ambulatory Surgical Centers; Physicians Clinics
Subsegment:
1) By Pacemakers: Single-Chamber Pacemakers; Dual-Chamber Pacemakers; Biventricular Pacemakers; Implantable Pacemakers2) By Defibrillators: Implantable Cardioverter Defibrillators (ICDs); External Defibrillators; Wearable Defibrillators; Automated External Defibrillators (AEDs)
3) By Cardiac Resynchronization Therapy (CRT): CRT-P (Pacemaker); CRT-D (Defibrillator); Bi-Ventricular Pacemakers
Key Companies Mentioned: Abbott Laboratories; Medtronic plc; Biotronik SE & Co KG; Cardiac Science Corporation; Schiller AG
Countries: Australia; China; India; Indonesia; Japan; South Korea; Bangladesh; Thailand; Vietnam; Malaysia; Singapore; Philippines; Hong Kong; New Zealand; USA; Canada; Mexico; Brazil; Chile; Argentina; Colombia; Peru; France; Germany; UK; Austria; Belgium; Denmark; Finland; Ireland; Italy; Netherlands; Norway; Portugal; Spain; Sweden; Switzerland; Russia; Czech Republic; Poland; Romania; Ukraine; Saudi Arabia; Israel; Iran; Turkey; UAE; Egypt; Nigeria; South Africa
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Cardiac Rhythm Management (CRM) Devices and Equipment market report include:- Abbott Laboratories
- Medtronic plc
- Biotronik SE & Co KG
- Cardiac Science Corporation
- Schiller AG
- Hill-Rom Holdings Inc.
- GE Healthcare
- Integer Holdings
- Neovasc Inc.
- Philips Healthcare
- Stryker Corporation
- Rochling Medical
- CCC Medical Devices
- LivaNova plc
- ZOLL Medical Corporation
- Koninklijke Philips N.V
- Applied Cardiac System
- Cook Medical
- Abiomed Inc.
- Berlin Heart GmbH
- Brotionik SE and Co. KG
- Jarvik Hearth Inc.
- MicroPort Scientific Corp
- ReliantHeart Inc.
- Shree Pacetronix Ltd.
- Shaanxi Qinming Medical Instrument Co. Ltd.
- AtriCure Inc.
- Boston Scientific Corporation
- Edwards Lifesciences Corporation
- Progetti Srl
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 17.15 Billion |
| Forecasted Market Value ( USD | $ 21.73 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |