The chimeric antigen receptor -T therapy market includes revenues earned by CAR-T therapy products and related services. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The chimeric antigen receptor -T therapy is a type of immunotherapy in which T-cells are taken from the patient’s blood are modified in a laboratory with the addition of a special protein receptor that grants T-cells the power to recognize as well as kill cancer cells easily, along with infusing the same back into that patient. This special protein receptor, known as the chimeric antigen receptor (CAR), attaches to a specific protein on a patient’s cancer cells. The infused cells multiply and prevail in the patient’s body as living drugs.
North America was the largest region in the global CAR-t therapy market in 2022. Western Europe was the second-largest region in the CAR-T therapy market. The regions covered in chimeric antigen receptor -T therapy market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, and Africa.
The main types of chimeric antigen receptor -T therapy is monotherapy and combination therapy. Monotherapy is used in the treatment of any disease with a single drug. The target antigens involved are CD19, CD22, and others that are used in various applications such as acute lymphoblastic leukemia, diffuse large b-cell lymphoma, follicular lymphoma, chronic lymphocytic leukemia, multiple myeloma, and others.
The CAR-T therapy market is driven by the increasing financial support provided by different organizations to promote the development and consumption of CAR-T therapy. The government and non-government organizations provide financial support to the companies in the CAR-T therapy market for research and development and the patients for their treatment of acute lymphoblastic leukemia (ALL). For instance, in December 2021, the University of California San Diego School of Medicine (CA, USA) was granted a funding of US$ 4.1 million for the development of novel chimeric antigen receptor (CAR-T) cell therapy. The grant was awarded by California Institute for Regenerative Medicine (CIRM; CA, USA) governing board.The financial support provided by different organizations towards CAR-T therapy positively drives the growth of the CAR-T therapy market.
The limitations on the application of CAR-T therapy limit the growth of the market. The limitations of CAR-T therapy include its failure to treat other types of cancer, side effects, and the high cost of treatment. CAR-T therapy is widely used as a treatment for a particular type of blood cancer and fails to treat other types of cancers such as lung cancer or breast cancer. Further, in many cases, the application of CAR-T therapy results in cytokine release syndrome (CRS). CRS is a severe flu-like condition causing high fever, nausea, chills, headache, rash, and troubled breathing. Further, the high cost limits the growth of the market. According to a report from Kaiser Health News, the per-patient cost of CAR-T cell therapy was more than $1 million in 2021. Thus, the growth of CAR-T therapy is restricted by the various limitations on the application of CAR-T therapy.
The companies in the CAR-T therapy market are conducting clinical trials to assess the ability of CAR-T therapy to treat multiple myeloma. Multiple myeloma is a type of white blood cell cancer where the cancer cells accumulate in the bone marrow and surrounds the healthy blood cells. CAR-T therapy is being tested as a treatment for multiple myeloma. CAR-T cells are modified to target the multiple myeloma causing cells to treat the relapsed or refractory multiple myeloma (RRMM). For instance, in December 2021, Novartis announced the introduction of T-Charge™, the company’s next-generation CAR-T platform that will serve as the foundation for various new investigational CAR-T cell therapies in the Novartis pipeline. The product was in the early clinical stage (Phase I).
In January 2020, Astellas Pharma, a Japan-based pharmaceuticals company acquired Xyphos for $120m(€107m). The acquisition would add five pre-clinical candidates to Astellas Pharma’s portfolio. Xyphos is a US-based CAR-T cell therapy developer.
The countries covered in the chimeric antigen receptor -T therapy market are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, and USA.
The market value is defined as the revenues that enterprises gain from goods and/or services sold within the specified market and geography through sales, grants, or donations in terms of currency (in USD ($) unless otherwise specified).
The revenues for a specified geography are consumption values - that is, they are revenues generated by organizations in the specified geography within the specified market, irrespective of where they are produced. It does not include revenues from resales either further along the supply chain or as part of other products.
The chimeric antigen receptor -T therapy market research report is one of a series of new reports that provides chimeric antigen receptor -T therapy market statistics, including chimeric antigen receptor -T therapy industry global market size, regional shares, competitors with a chimeric antigen receptor -T therapy market share, detailed chimeric antigen receptor -T therapy market segments, market trends and opportunities, and any further data you may need to thrive in the chimeric antigen receptor -T therapy industry. This chimeric antigen receptor -T therapy market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Major players in the chimeric antigen receptor -T therapy market are Novartis AG, Kite Pharma, Pfizer Inc, Juno Therapeutics, Celgene Corporation, CARsgen Therapeutics, Sorrento Therapeutics, Legend Biotech, Mustang Bio, and Immune Therapeutics.
This product will be delivered within 3-5 business days.
Table of Contents
1. Executive Summary2. CAR-T Therapy Market Characteristics
3. CAR-T Therapy Market Trends And Strategies
4. CAR-T Therapy Market- Macro Economic Scenario
4.1 COVID-19 Impact On CAR-T Therapy Market
4.2 Ukraine-Russia War Impact On CAR-T Therapy Market
4.3 Impact Of High Inflation On CAR-T Therapy Market
5. CAR-T Therapy Market Size And Growth
5.1. Global CAR-T Therapy Historic Market, 2017-2022, $ Billion
5.1.1. Drivers Of The Market
5.1.2. Restraints On The Market
5.2. Global CAR-T Therapy Forecast Market, 2022-2027F, 2032F, $ Billion
5.2.1. Drivers Of The Market
5.2.2. Restraints On the Market
6. CAR-T Therapy Market Segmentation
6.1. Global CAR-T Therapy Market, Segmentation By Type, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
- Monotherapy
- Combination Therapy
- CD19
- CD22
- Other Target Antigen
- Acute Lymphoblastic Leukemia
- Diffuse Large B-Cell Lymphoma
- Follicular Lymphoma
- Chronic Lymphocytic Leukemia
- Multiple Myeloma
- Other Applications
7.1. Global CAR-T Therapy Market, Split By Region, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
7.2. Global CAR-T Therapy Market, Split By Country, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
8. Asia-Pacific CAR-T Therapy Market
8.1. Asia-Pacific CAR-T Therapy Market Overview
- Region Information, Impact Of COVID-19, Market Information, Background Information, Government Initiatives, Regulations, Regulatory Bodies, Major Associations, Taxes Levied, Corporate Tax Structure, Investments, Major Companies
9. China CAR-T Therapy Market
9.1. China CAR-T Therapy Market Overview
9.2. China CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F,$ Billion
10. India CAR-T Therapy Market
10.1. India CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
11. Japan CAR-T Therapy Market
11.1. Japan CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
12. Australia CAR-T Therapy Market
12.1. Australia CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
13. Indonesia CAR-T Therapy Market
13.1. Indonesia CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
14. South Korea CAR-T Therapy Market
14.1. South Korea CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
15. Western Europe CAR-T Therapy Market
15.1. Western Europe CAR-T Therapy Market Overview
15.2. Western Europe CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
16. UK CAR-T Therapy Market
16.1. UK CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
17. Germany CAR-T Therapy Market
17.1. Germany CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
18. France CAR-T Therapy Market
18.1. France CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
19. Eastern Europe CAR-T Therapy Market
19.1. Eastern Europe CAR-T Therapy Market Overview
19.2. Eastern Europe CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
20. Russia CAR-T Therapy Market
20.1. Russia CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
21. North America CAR-T Therapy Market
21.1. North America CAR-T Therapy Market Overview
21.2. North America CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
22. USA CAR-T Therapy Market
22.1. USA CAR-T Therapy Market Overview
22.2. USA CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
23. South America CAR-T Therapy Market
23.1. South America CAR-T Therapy Market Overview
23.2. South America CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
24. Brazil CAR-T Therapy Market
24.1. Brazil CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
25. Middle East CAR-T Therapy Market
25.1. Middle East CAR-T Therapy Market Overview
25.2. Middle East CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
26. Africa CAR-T Therapy Market
26.1. Africa CAR-T Therapy Market Overview
26.2. Africa CAR-T Therapy Market, Segmentation By Application, Historic and Forecast, 2017-2022, 2022-2027F, 2032F, $ Billion
27. CAR-T Therapy Market Competitive Landscape And Company Profiles
27.1. CAR-T Therapy Market Competitive Landscape
27.2. CAR-T Therapy Market Company Profiles
27.2.1. Novartis AG
27.2.1.1. Overview
27.2.1.2. Products and Services
27.2.1.3. Strategy
27.2.1.4. Financial Performance
27.2.2. Kite Pharma
27.2.2.1. Overview
27.2.2.2. Products and Services
27.2.2.3. Strategy
27.2.2.4. Financial Performance
27.2.3. Pfizer Inc
27.2.3.1. Overview
27.2.3.2. Products and Services
27.2.3.3. Strategy
27.2.3.4. Financial Performance
27.2.4. Juno Therapeutics
27.2.4.1. Overview
27.2.4.2. Products and Services
27.2.4.3. Strategy
27.2.4.4. Financial Performance
27.2.5. Celgene Corporation
27.2.5.1. Overview
27.2.5.2. Products and Services
27.2.5.3. Strategy
27.2.5.4. Financial Performance
28. CAR-T Therapy Pipeline Analysis
29. Key Mergers And Acquisitions In The CAR-T Therapy Market
30. CAR-T Therapy Market Future Outlook and Potential Analysis
31. Appendix
31.1. Abbreviations
31.2. Currencies
31.3. Historic And Forecast Inflation Rates
31.4. Research Inquiries
31.5. About the Publisher
31.6. Copyright And Disclaimer
Executive Summary
CAR-T Therapy Pipeline Analysis Global Market Report 2023 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on car-t therapy pipeline analysis market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase
- Gain a truly global perspective with the most comprehensive report available on this market covering 50+ geographies.
- Understand how the market has been affected by the coronavirus and how it is responding as the impact of the virus abates.
- Assess the Russia - Ukraine war’s impact on agriculture, energy and mineral commodity supply and its direct and indirect impact on the market.
- Measure the impact of high global inflation on market growth.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
Scope
Markets Covered
1) By Type: Monotherapy; Combination Therapy2) By Target Antigen: CD19; CD22; Other Target Antigens
3) By Application: Acute Lymphoblastic Leukemia; Diffuse Large B-Cell Lymphoma; Follicular Lymphoma; Chronic Lymphocytic Leukemia; Multiple Myeloma; Other Applications
Companies Mentioned: Novartis AG; Kite Pharma; Pfizer Inc.; Juno Therapeutics
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita,
Data segmentations: country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Novartis AG
- Kite Pharma
- Pfizer Inc.
- Juno Therapeutics
- Celgene Corporation
- CARsgen Therapeutics
- Sorrento Therapeutics
- Legend Biotech
- Mustang Bio
- Immune Therapeutics
- Atara Biotherapeutics
- Aurora Biopharma Inc.
- Eureka Therapeutics
- Autolus
- TILT Biotherapeutics
- Fortress Biotech.
- Autolus Therapeutics PLC
- bluebird bio
- Carina Biotech
- CARTherics
- Endocyte, Inc.
- F1 Oncology, Inc.
- Fate Therapeutics Inc.
- Gilead
- Oxford BioMedica PLC.
- PeproMene Bio Inc.
- Tessa Therapeutics Pte Ltd.
- TILT Biotherapeutics Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 175 |
| Published | February 2023 |
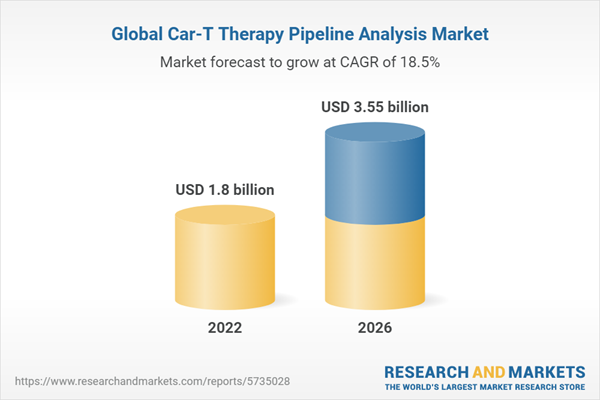
| Forecast Period | 2022 - 2026 |
| Estimated Market Value ( USD | $ 1.8 billion |
| Forecasted Market Value ( USD | $ 3.55 billion |
| Compound Annual Growth Rate | 18.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 28 |