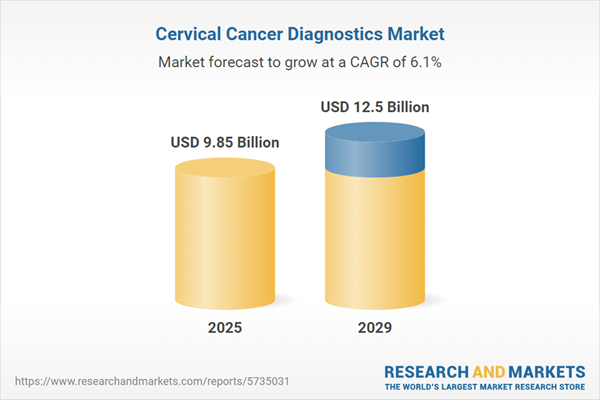
The cervical cancer diagnostics market size has grown strongly in recent years. It will grow from $9.27 billion in 2024 to $9.85 billion in 2025 at a compound annual growth rate (CAGR) of 6.2%. The growth in the historic period can be attributed to increased awareness and screening programs, hpv vaccination, technological advancements, aging population.
The cervical cancer diagnostics market size is expected to see strong growth in the next few years. It will grow to $12.5 billion in 2029 at a compound annual growth rate (CAGR) of 6.1%. The growth in the forecast period can be attributed to increasing hpv-related cancers, emerging markets, molecular testing advances, telemedicine and remote screening. Major trends in the forecast period include hpv testing, liquid-based cytology, automation and digital pathology, screen-and-treat programs.
The increasing adoption of cervical cancer diagnostic tests for early detection is expected to drive the growth of the cervical cancer diagnostics market. Rising awareness among women about cervical cancer, along with global cancer organizations and governments' emphasis on early testing for detection and prevention, contributes to the market's expansion. For example, in July 2024, the Centers for Disease Control and Prevention (CDC), a US-based government agency, reported 12,536 new cases of cervical cancer in the United States in 2021, while in 2022, the disease led to 4,051 fatalities among women. The growing adoption of cervical cancer diagnostic tests and the focus on early diagnosis are key factors driving the growth of the cervical cancer diagnostics market.
The increasing prevalence of cervical cancer is anticipated to be a key driver of growth in the cervical cancer diagnostics market. Cervical cancer, originating in the cervix, the narrow lower portion of the uterus, is a serious health concern. Diagnostic tests for cervical cancer play a pivotal role in early detection, accurate diagnosis, and effective treatment. For instance, estimates from Cancer.net, the patient information website of the American Society of Clinical Oncology (ASCO), suggest that approximately 13,960 women in the United States will be diagnosed with invasive cervical cancer. Consequently, the rising incidence of cervical cancer is expected to underpin the growth of the cervical cancer diagnostics market.
Leading companies in the cervical cancer diagnostics market are emphasizing the development of technologically advanced solutions, such as HPV self-collection methods, to enhance screening accessibility and improve early detection rates. HPV self-collection solutions enable individuals to independently collect samples for testing the human papillomavirus (HPV), a primary cause of cervical cancer. For instance, in May 2024, F. Hoffmann-La Roche AG, a pharmaceutical company based in Switzerland, secured FDA approval for its HPV self-collection solution. This innovation marks a significant milestone in cervical cancer screening by improving accessibility, particularly for underserved populations. The solution allows women to privately collect vaginal samples for testing using Roche’s Cobas molecular HPV test, aligning with the World Health Organization’s objective of eradicating cervical cancer by 2030. The FDA approval follows the successful IMPACT trial, which validated the solution’s effectiveness across various demographic groups.
Prominent players in the cervical cancer diagnostics industry are prioritizing the development of advanced digital diagnostic systems to enhance screening accessibility and early detection capabilities. Digital diagnostic systems leverage cutting-edge technologies, including artificial intelligence (AI) and digital tools, to support the detection, diagnosis, and management of cervical cancer. For example, in February 2024, Hologic, Inc., a US-based health technology firm, obtained FDA clearance for its Genius Digital Diagnostics System. This groundbreaking solution integrates the Genius Cervical AI algorithm with advanced volumetric imaging technology to significantly improve the detection of precancerous lesions and cervical cancer cells. By reducing false negatives by 28% compared to traditional methods, the system enhances early detection and patient outcomes. It digitizes cytology slides and employs AI to guide cytologists and pathologists in identifying areas needing further analysis, thereby optimizing workflow efficiency and facilitating remote collaboration among healthcare professionals.
In February 2022, BD (Becton, Dickinson, and Company), a manufacturer of medical devices and reagents, acquired Cytognos for an undisclosed amount. BD's acquisition of Cytognos strengthens its commitment to chronic disease management, expanding its diagnostic portfolio, immune assessment tests, and informatics to gain deeper insights into the immune system, immune response, and Minimal Residual Disease (MRD). Cytognos, a Spain-based company, specializes in providing clinical flow cytometry solutions used in cervical cancer diagnostics.
Cervical cancer is a form of squamous cell carcinoma that develops in the cervix, which is the lower part of the uterus. Diagnostic devices for cervical cancer are employed to detect cancer affecting the cervix.
The primary types of cervical cancer diagnostics include pap smear tests, HPV tests, colposcopy, biopsy, endocervical curettage, and other diagnostic procedures. A pap smear test is a method used for the detection of cervical cancer in women. This diagnostic process encompasses age groups such as those under 21, those aged 21 to 29, those aged 30 to 65, and those over 65. These diagnostics are utilized by various end-users, including hospitals, specialty clinics, cancer and radiation therapy centers, as well as diagnostic centers.
The cervical cancer diagnostics market research report is one of a series of new reports that provides cervical cancer diagnostics market statistics, including cervical cancer diagnostics industry global market size, regional shares, competitors with a cervical cancer diagnostics market share, detailed cervical cancer diagnostics market segments, market trends and opportunities, and any further data you may need to thrive in the cervical cancer diagnostics industry. This cervical cancer diagnostics market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Major companies operating in the cervical cancer diagnostics market include Abbott Laboratories, Becton Dickinson and Company (BD), Bio-Rad Laboratories Inc., F. Hoffmann-La Roche Ltd., Hologic Inc., QIAGEN N.V., Quest Diagnostics Incorporated, Siemens Healthineers AG, Zilico Ltd., Guided Therapeutics Inc., Thermo Fisher Scientific Inc., Carl Zeiss AG, OncoHealth Corporation, Arbor Vita Corporation, Micromedic Technologies Ltd., Trovagene Inc., Roche Diagnostics, Nucleix Ltd., BGI Genomics Co. Ltd., Cepheid, Exact Sciences Corporation, Guardant Health Inc., Invitae Corporation, Natera Inc., NeoGenomics Laboratories Inc., PerkinElmer Inc., Sysmex Corporation, Veracyte Inc., Zymo Research Corporation.
North America was the largest region in the cervical cancer diagnostics market in 2024 Asia-Pacific was the second largest region in the cervical cancer diagnostics market. The regions covered in the cervical cancer diagnostics market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the cervical cancer diagnostics market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
The cervical cancer diagnostics market consists of sales of analyzer, reagents, and others that are used for the diagnosis of cervical cancer. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Cervical Cancer Diagnostics Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on cervical cancer diagnostics market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for cervical cancer diagnostics ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The cervical cancer diagnostics market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Diagnostic Test: Pap Smear Test; HPV Test; Colposcopy; Biopsy and Endocervical Curettage; Other Diagnostic Tests2) By Age Group: Below 21; Age between 21 to 29; Age between 30 to 65; Above 65
3) By End User: Hospitals; Specialty Clinics; Cancer and Radiation Therapy Centers; Diagnostic Centers
Subsegments:
1) By Pap Smear Test: Conventional Pap Smear; Liquid-Based Pap Smear2) By HPV Test: DNA-Based HPV Test; RNA-Based HPV Test; Hybrid Capture HPV Test; Other HPV Tests
3) By Colposcopy: Standard Colposcopy; Digital Colposcopy; Optical Colposcopy
4) By Biopsy and Endocervical Curettage: Punch Biopsy; Endocervical Curettage (ECC); Cone Biopsy; Colposcopic Biopsy
5) By Other Diagnostic Tests: Visual Inspection With Acetic Acid (VIA); Cervical Cytology Testing; Blood Tests; Other Specialty Tests
Key Companies Mentioned: Abbott Laboratories; Becton Dickinson and Company (BD); Bio-Rad Laboratories Inc.; F. Hoffmann-La Roche Ltd.; Hologic Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Cervical Cancer Diagnostics market report include:- Abbott Laboratories
- Becton Dickinson and Company (BD)
- Bio-Rad Laboratories Inc.
- F. Hoffmann-La Roche Ltd.
- Hologic Inc.
- QIAGEN N.V.
- Quest Diagnostics Incorporated
- Siemens Healthineers AG
- Zilico Ltd.
- Guided Therapeutics Inc.
- Thermo Fisher Scientific Inc.
- Carl Zeiss AG
- OncoHealth Corporation
- Arbor Vita Corporation
- Micromedic Technologies Ltd.
- Trovagene Inc.
- Roche Diagnostics
- Nucleix Ltd.
- BGI Genomics Co. Ltd.
- Cepheid
- Exact Sciences Corporation
- Guardant Health Inc.
- Invitae Corporation
- Natera Inc.
- NeoGenomics Laboratories Inc.
- PerkinElmer Inc.
- Sysmex Corporation
- Veracyte Inc.
- Zymo Research Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 175 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 9.85 Billion |
| Forecasted Market Value ( USD | $ 12.5 Billion |
| Compound Annual Growth Rate | 6.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |