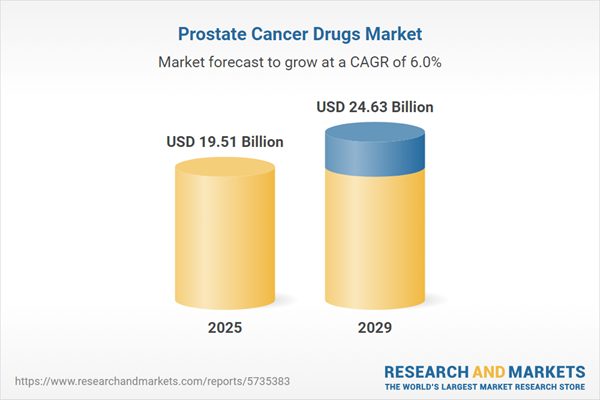
The prostate cancer drugs market size is expected to see strong growth in the next few years. It will grow to $24.63 billion in 2029 at a compound annual growth rate (CAGR) of 6%. The growth in the forecast period can be attributed to growing incidence, emerging markets, supportive government policies, r&d investments. Major trends in the forecast period include precision medicine, immunotherapy and targeted therapies, personalized medicine, clinical trials and drug pipeline, combination therapies, biomarker-driven treatments, telemedicine and remote monitoring.
The forecast of 6% growth over the next five years reflects a modest reduction of 0.3% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Trade escalations are likely to burden U.S. urology clinics by driving up the cost of androgen receptor inhibitors and metastatic castration-resistant prostate cancer therapies sourced from the UK and Japan, exacerbating oncology treatment costs and increasing specialty drug burdens. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The prostate cancer drugs market is significantly driven by the increasing male geriatric population. Prostate cancer predominantly affects men aged above 60, and the geriatric population is anticipated to grow by 21.6% by 2040, as indicated by the Administration for Community Living (ACL), a US-based government organization. Prostate cancer incidence is higher in older men, with approximately 6 out of 10 cases diagnosed in men aged 65 or older within the geriatric population, according to the American Cancer Society's January 2022 report. Thus, the growing population of elderly men is a key factor fueling the expansion of the prostate cancer drugs market.
The rising cancer incidence is expected to be a driving force behind the future growth of the prostate cancer drugs market. Cancer is characterized by the uncontrolled growth and division of abnormal cells in the body, and prostate cancer drugs play a crucial role in targeting specific cellular changes that promote cancer cell growth. This differs from traditional chemotherapy and hormone therapy. In January 2023, the American Cancer Society estimated approximately 288,300 new cases of prostate cancer and about 34,700 deaths related to the disease. One in eight men is projected to be diagnosed with prostate cancer during their lifetime. Consequently, the increasing incidence of cancer is a significant driver for the prostate cancer drugs market.
An emerging trend in the market is the adoption of combination therapy for the treatment of prostate cancer. This approach is particularly useful in cases where monotherapy proves ineffective. Market companies are increasingly investing in combination therapy, such as the use of XTANDI (enzalutamide) alongside androgen deprivation therapy (ADT) for non-metastatic castration-resistant prostate cancer. This combination therapy has demonstrated a significant 71% reduction in the risk of metastasis or death compared to ADT alone. Similar combination therapies include the use of radiation therapy and ADT for men with recurrent prostate cancer and the use of the chemotherapy drug docetaxel (Taxotere) in conjunction with ADT for prostate cancer treatment.
Major companies in the prostate cancer drugs market are striving to introduce new products and gain approvals from regulatory agencies to maximize their market revenues. The process of drug development and approval is intricate and time-consuming, necessitating a thorough understanding of regulatory controls and marketing pathways to ensure safety and effectiveness. For instance, in June 2023, AstraZeneca PLC and Merck & Co. Inc. announced the approval of LYNPARZA (olaparib) Plus Abiraterone and Prednisone or Prednisolone for treating adult patients with BRCA-mutated metastatic castration-resistant prostate cancer (mCRPC) by the United States Food and Drug Administration (USFDA). The approval was based on the significant improvements in radiographic progression-free survival (rPFS) and overall survival (OS) demonstrated by LYNPARZA plus abi/pred in comparison to abi/pred alone, as observed in the Phase 3 PROpel trial.
In March 2024, Johnson & Johnson, a US-based pharmaceutical company, acquired Ambrx Biopharma Inc. for an undisclosed sum. This acquisition enables Johnson & Johnson to advance the development of targeted oncology therapies by leveraging Ambrx's proprietary ADC technology, which integrates highly specific monoclonal antibodies with powerful chemotherapeutic agents. This innovative approach enhances the precision of cancer treatment, reducing common chemotherapy side effects and revolutionizing the treatment landscape for solid tumors, such as prostate cancer. Ambrx Biopharma Inc. is a biotechnology company based in the United States, specializing in the creation of groundbreaking therapies, including drugs for prostate cancer.
Major companies operating in the prostate cancer drugs market include Astellas Pharma Inc., AstraZeneca PLC, Johnson & Johnson, Sanofi S.A., Bayer AG, F. Hoffmann-La Roche AG, Abbott Laboratories, Pfizer Inc., Novartis International AG, F. Hoffmann-La Roche Ltd., Tolmar Inc., AbbVie Inc., Siemens Healthineers AG, Genomic Health Inc., OPKO Health Inc., Siemens Healthcare GmbH, MDxHealth SA, Myriad Genetics Inc., Janssen Biotech Inc., Clovis Oncology Inc., Merck Sharp & Dohme Corp., Dendreon Corporation, Ferring Pharmaceuticals Inc., GlaxoSmithKline PLC, Ipsen Biopharmaceuticals Inc., Merck & Co. Inc., Eli Lilly and Company Inc., Bausch Health Companies Inc., Bristol-Myers Squibb Company, Takeda Pharmaceutical Company Limited, Novartis AG, Amgen Inc., Biogen Inc., Celgene Corporation, Gilead Sciences Inc., Regeneron Pharmaceuticals Inc., Agios Pharmaceuticals Inc., Exelixis Inc.
North America was the largest region in the prostate cancer drugs market in 2024. Middle East is expected to be the fastest-growing region in the prostate cancer drugs market during the forecast period. The regions covered in the prostate cancer drugs market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the prostate cancer drugs market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The prostate cancer drugs market research report is one of a series of new reports that provides prostate cancer drugs market statistics, including prostate cancer drugs industry global market size, regional shares, competitors with a prostate cancer drugs market share, detailed prostate cancer drugs market segments, market trends and opportunities, and any further data you may need to thrive in the prostate cancer drugs industry. This prostate cancer drugs market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Prostate cancer drugs are pharmaceuticals employed for both the prevention and treatment of prostate cancer. Prostate cancer is among the most prevalent malignancies, characterized by the uncontrolled proliferation of cells in the prostate gland of men, which is responsible for producing seminal fluid for nourishing and transporting sperm. Potential treatment options for prostate cancer encompass hormonal therapy, chemotherapy, immunotherapy, and targeted therapy.
The distinct categories of prostate cancer drugs revolve around hormone-sensitive prostate cancer and hormone-refractory prostate cancer. Hormone-refractory prostate cancer (HRPC) represents a form of prostate cancer that no longer responds to hormone therapy, even to more recent treatment approaches. Various therapeutic methods encompass hormonal therapy, chemotherapy, immunotherapy, and targeted therapy and are implemented across diverse healthcare sectors, including hospitals, clinics, and other medical facilities.
The prostate cancer drugs market consists of sales of Abiraterone Acetate, Apalutamide, and Bicalutamide. Values in this market are factory gate values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Prostate Cancer Drugs Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on prostate cancer drugs market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for prostate cancer drugs? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The prostate cancer drugs market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Hormone Sensitive Prostate Cancer; Hormone Refractory Prostate Cancer2) By Therapy: Hormonal Therapy; Chemotherapy; Immunotherapy; Targeted Therapy
3) By End User: Hospitals; Clinics; Other End-Users
Subsegments:
1) By Hormone Sensitive Prostate Cancer: Early-Stage Prostate Cancer; Locally Advanced Prostate Cancer; Metastatic Prostate Cancer2) By Hormone Refractory Prostate Cancer: Castration-Resistant Prostate Cancer (CRPC); Metastatic Castration-Resistant Prostate Cancer (mCRPC)
Companies Mentioned: Astellas Pharma Inc.; AstraZeneca PLC; Johnson & Johnson; Sanofi S.A.; Bayer AG; F. Hoffmann-La Roche AG; Abbott Laboratories; Pfizer Inc.; Novartis International AG; F. Hoffmann-La Roche Ltd.; Tolmar Inc.; AbbVie Inc.; Siemens Healthineers AG; Genomic Health Inc.; OPKO Health Inc.; Siemens Healthcare GmbH; MDxHealth SA; Myriad Genetics Inc.; Janssen Biotech Inc.; Clovis Oncology Inc.; Merck Sharp & Dohme Corp.; Dendreon Corporation; Ferring Pharmaceuticals Inc.; GlaxoSmithKline plc; Ipsen Biopharmaceuticals Inc.; Merck & Co. Inc.; Eli Lilly and Company Inc.; Bausch Health Companies Inc.; Bristol-Myers Squibb Company; Takeda Pharmaceutical Company Limited; Novartis AG; Amgen Inc.; Biogen Inc.; Celgene Corporation; Gilead Sciences Inc.; Regeneron Pharmaceuticals Inc.; Agios Pharmaceuticals Inc.; Exelixis Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Prostate Cancer Drugs market report include:- Astellas Pharma Inc.
- AstraZeneca PLC
- Johnson & Johnson
- Sanofi S.A.
- Bayer AG
- F. Hoffmann-La Roche AG
- Abbott Laboratories
- Pfizer Inc.
- Novartis International AG
- F. Hoffmann-La Roche Ltd.
- Tolmar Inc.
- AbbVie Inc.
- Siemens Healthineers AG
- Genomic Health Inc.
- OPKO Health Inc.
- Siemens Healthcare GmbH
- MDxHealth SA
- Myriad Genetics Inc.
- Janssen Biotech Inc.
- Clovis Oncology Inc.
- Merck Sharp & Dohme Corp.
- Dendreon Corporation
- Ferring Pharmaceuticals Inc.
- GlaxoSmithKline plc
- Ipsen Biopharmaceuticals Inc.
- Merck & Co. Inc.
- Eli Lilly and Company Inc.
- Bausch Health Companies Inc.
- Bristol-Myers Squibb Company
- Takeda Pharmaceutical Company Limited
- Novartis AG
- Amgen Inc.
- Biogen Inc.
- Celgene Corporation
- Gilead Sciences Inc.
- Regeneron Pharmaceuticals Inc.
- Agios Pharmaceuticals Inc.
- Exelixis Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 19.51 Billion |
| Forecasted Market Value ( USD | $ 24.63 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 39 |