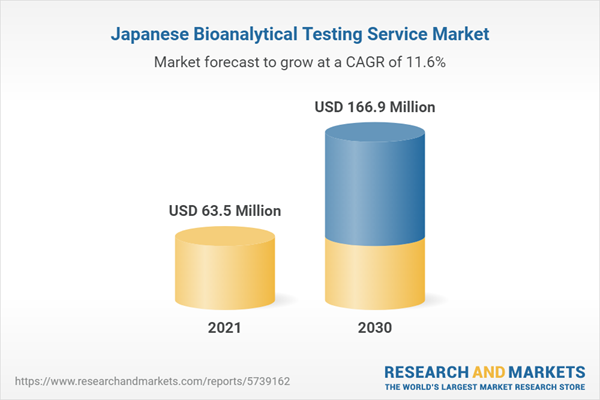
The Japan bioanalytical testing service market held a market value of USD 63.5 million in 2021 and is projected to reach USD 166.9 million by the year 2030. The market is anticipated to register a CAGR of 11.6% during the forecast period.Japan bioanalytical testing service market is anticipated to grow at a CAGR of 11.6% during the forecast period 2022 -2030
A growing emphasis on the creation of sophisticated bioanalytical technologies for improved testing is anticipated to support market expansion. The demand for certain testing methods has increased as a result of the introduction of biosimilars, combination products, and other novel medicines. The global pharmaceutical industry's supply chain has been affected by the coronavirus epidemic. However, the outbreak had a positive impact on the market for bioanalytical testing services. Key stakeholders' R&D and manufacturing operations were expanded as a result of the epidemic, which increased demand for these services.
The area of pharmacology that deals with what happens to a drug after administration is called pharmacokinetics (PK). The bulk of outsourcing CROs are using cutting-edge techniques to quantify PK parameters. For instance, SGS supplies Dry Blood Spot (DBS) technology, which has many benefits over conventional approaches. The advantages of the DBS approach are the lack of post-collection processing, reduced sample requirements, simple sample storage & shipment, and low biohazard concerns. Therefore, it is projected that the introduction and acceptance of cutting-edge technology for pharmacokinetics research will accelerate the segment's growth.
Several businesses are increasing their capacity to offer testing services for fresh viral risks by utilising quick and sophisticated testing platforms. According to estimates, the market will benefit from these expansions.
Growth Influencers:
Increasing Preference for Outsourcing Analytical Testing
A number of biopharmaceutical firms use outsourcing services for bioanalytical testing to develop drugs and validate assays at both the preclinical and clinical phases, contributing to the market's expansion. The necessity for early detection and comprehensive screening is being driven by the widespread transmission of the coronavirus disease and the dearth of curative medications and vaccinations. Additionally, this has improved the use of bioanalytical testing services for point-of-care diagnostic and reduced the infection's social burden.Rising adoption of the Quality by Design approach
The pharmaceutical industry is encouraged by quality by design (QbD) to use risk management and science-based manufacturing approaches to increase process and product understanding and ensure product quality. The ICH actively promotes QbD. QbD guarantees efficient medication supply and enhanced production performance. The idea is founded on the idea of continuous development and the growing demand for businesses to expand their knowledge of their products. As a result, this methodology enables businesses to continuously enhance their development strategies while also making adjustments. As a result, this fuels market expansion.Segments Overview:
The Japan bioanalytical testing service market is segmented into test type, molecule type, application, and end user.By Test Type
- Cell-based Assays
- Virology Testing
- Species-specific Viral PCR Assays
- Method Development Optimization and Validation
- Serology, Immunogenicity, and Neutralizing Antibodies
- Biomarker Assays
- Pharmacokinetic Testing
- Others
By Molecule Type
- Small Molecule Bioanalysis
- Large Molecule Bioanalysis
- Others
By Application
- Oncology
- Neurology
- Infectious Diseases
- Gastroenterology
- Cardiology
- Others
By End User
- Pharma & Biotechnology Companies
- Contract Research Organizations
- Others
Country Overview
This expansion is primarily attributed to the increased demand for outsourced services in areas that are still developing. In this country, numerous delivery centres often support outsourcing services. Due to the increasing strategic innovations made by the market players, the Japan bioanalytical testing service industry is predicted to grow strongly over the projected period. Additionally, QbD research is expanding, which is fostering the expansion. For example, the design of experiments (DOE) is useful for categorising the impact of important interactions. It is simpler to carry out risk mitigation steps and prevent batch rejection when crucial factors can be recognised in advance. Additionally, DOE is effectively utilised to identify limitations for a variety of process parameters and identify the best process conditions.Competitive Landscape
Key players operating in the Japan bioanalytical testing service market include Medpace, WuXi AppTec, Eurofins Scientific, ICON plc, PRA HEALTH SCIENCES (Takeda), inVentiv Health, Intertek Group, IQVIA, Medpace, Laboratory Corporation of America, PPD, Inc., SGS SA, Charles River Laboratories International, Inc., Syneos Health, among others.The key ten players in the market hold more than 60% of the market share. These players are involved in strategies, such as mergers, acquisitions to sustain in the market. For instance, Intertek Group obtained 61.3%, 21.6%, and 17.1% revenue through its business segments of products, trade and resources, respectively.
The Japan bioanalytical testing service market report provides insights on the below pointers:
- Market Penetration: Provides comprehensive information on the market offered by the prominent players
- Market Development: The report offers detailed information about lucrative emerging markets and analyzes penetration across mature segments of the markets
- Market Diversification: Provides in-depth information about untapped geographies, recent developments, and investments
- Competitive Landscape Assessment: Mergers & acquisitions, certifications, product launches in the Japan Bioanalytical testing service market have been provided in this research report. In addition, the report also emphasizes the SWOT analysis of the leading players
- Product Development & Innovation: The report provides intelligent insights on future technologies, R&D activities, and breakthrough product developments
- Pricing Analysis: Pricing analysis of various components used in the manufacturing
- Manufacturing Cost Analysis: Cost-share of various components in bioanalytical testing service
- Advancement in Bioanalytical Techniques for Biotherapeutics
The Japan bioanalytical testing service market report answers questions such as:
- What is the market size and forecast of the Japan bioanalytical testing service market?
- What are the inhibiting factors and impact of COVID-19 on the Japan bioanalytical testing service market during the assessment period?
- Which are the products/segments/areas to invest in over the assessment period in the Japan bioanalytical testing service market?
- What is the competitive strategic window for opportunities in the Japan bioanalytical testing service market?
- What are the technology trends and regulatory frameworks in the Japan bioanalytical testing service market?
- What is the market share of the leading players in the Japan bioanalytical testing service market?
- What modes and strategic moves are considered favorable for entering the Japan bioanalytical testing service market?
Table of Contents
Chapter 1. Research Framework1.1. Research Objective
1.2. Product Overview
1.3. Market Segmentation
Chapter 2. Research Methodology
2.1. Qualitative Research
2.1.1. Primary & Secondary Sources
2.2. Quantitative Research
2.2.1. Primary & Secondary Sources
2.3. Breakdown of Primary Research Respondents, By Region
2.4. Assumption for the Study
2.5. Market Size Estimation
2.6. Data Triangulation
Chapter 3. Executive Summary: Japan Bioanalytical Testing Service Market
Chapter 4. Japan Bioanalytical Testing Service Market Overview
4.1. Industry Value Chain Analysis
4.1.1. Sample Collection
4.1.2. Sample Preparation & Validation
4.1.3. Service Provider
4.1.4. End User
4.2. Industry Outlook
4.2.1. Advancement in bioanalytical Techniques for Biotherapeutics
4.3. PESTLE Analysis
4.4. Porter's Five Forces Analysis
4.4.1. Bargaining Power of Suppliers
4.4.2. Bargaining Power of Buyers
4.4.3. Threat of Substitutes
4.4.4. Threat of New Entrants
4.4.5. Degree of Competition
4.5. Market Dynamics and Trends
4.5.1. Growth Drivers
4.5.2. Restraints
4.5.3. Challenges
4.5.4. Key Trends
4.6. Covid-19 Impact Assessment on Market Growth Trend
4.7. Market Growth and Outlook
4.7.1. Market Revenue Estimates and Forecast (US$ Mn), 2017 - 2030
4.7.2. Price Trend Analysis, By Type Test
4.8. Competition Dashboard
4.8.1. Market Concentration Rate
4.8.2. Company Market Share Analysis (Value %), 2020
4.8.3. Competitor Mapping
Chapter 5. Bioanalytical Testing Service Market Analysis, By Test Type
5.1. Key Insights
5.2. Market Size and Forecast, 2017 - 2030 (US$ Mn)
5.2.1. Cell-based Assays
5.2.1.1. Bacterial cell-based assays
5.2.1.2. Viral cell-based assays
5.2.2. Virology Testing
5.2.2.1. In Vitro Virology Testing
5.2.2.2. In Vivo Virology Testing
5.2.3. Species-specific Viral PCR Assays
5.2.4. Method Development Optimization and Validation
5.2.5. Serology, Immunogenicity, and Neutralizing Antibodies
5.2.6. Biomarker Assays
5.2.6.1. LBA/LC-MS/MS
5.2.7. Pharmacokinetic Testing
5.2.8. Others
Chapter 6. Bioanalytical Testing Service Market Analysis, By Molecule Type
6.1. Key Insights
6.2. Market Size and Forecast, 2017 - 2030 (US$ Mn)
6.2.1. Small Molecule Bioanalysis
6.2.2. Large Molecule Bioanalysis
6.2.3. Others
Chapter 7. Bioanalytical Testing Service Market Analysis, By Application
7.1. Key Insights
7.2. Market Size and Forecast, 2017 - 2030 (US$ Mn)
7.2.1. Oncology
7.2.2. Neurology
7.2.3. Infectious Diseases
7.2.4. Gastroenterology
7.2.5. Cardiology
7.2.6. Others
Chapter 8. Bioanalytical Testing Service Market Analysis, By End User
8.1. Key Insights
8.2. Market Size and Forecast, 2017 - 2030 (US$ Mn)
8.2.1. Pharma & Biotechnology Companies
8.2.2. Contract Research Organizations
8.2.3. Others
Chapter 9. Company Profile (Company Overview, Financial Matrix, Key Product landscape, Key Personnel, Key Competitors, Contact Address, and Business Strategy Outlook)
9.1. Medpace
9.2. PRA HEALTH SCIENCES (Takeda)
9.3. Eurofins Scientific
9.4. ICON plc
9.5. Intertek Group
9.6. inVentiv Health
9.7. IQVIA
9.8. Laboratory Corporation of America
9.9. Medpace
9.10. PPD, Inc.
9.11. Charles River Laboratories International, Inc.
9.12. SGS SA
9.13. Syneos Health
9.14. WuXi AppTec
9.15. Other Prominent Players
Executive Summary
Due to the expanding use of bioanalytical testing services in cancer, gastroenterology, and other medical end use fields, the Japan bioanalytical testing service market is anticipated to experience considerable expansion over the forecast period of 2022 to 2030. The market's growth is being constrained by excessive technical expenditure and insufficient technological expertise. By 2030, the market is expected to reach USD 166.9 million. In addition to that, the major market competitors are constantly engaged in strategic alliances to advance applications across numerous industries, along with joint ventures, partnerships, and service launches. Based on test type, molecule type, application, and end user, the market is divided respectively.Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Medpace
- WuXi AppTec
- Eurofins Scientific
- ICON plc
- PRA HEALTH SCIENCES (Takeda)
- inVentiv Health
- Intertek Group
- IQVIA
- Laboratory Corporation of America
- PPD, Inc.
- SGS SA
- Charles River Laboratories International, Inc.
- Syneos Health
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 109 |
| Published | December 2022 |
| Forecast Period | 2021 - 2030 |
| Estimated Market Value ( USD | $ 63.5 Million |
| Forecasted Market Value ( USD | $ 166.9 Million |
| Compound Annual Growth Rate | 11.6% |
| Regions Covered | Japan |