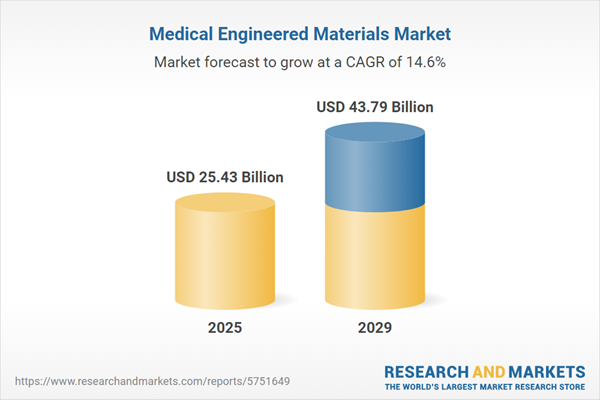
The medical engineered materials market size has grown rapidly in recent years. It will grow from $22.12 billion in 2024 to $25.43 billion in 2025 at a compound annual growth rate (CAGR) of 15%. The growth in the historic period can be attributed to aging population, regulatory compliance, infection control, materials for implants, tissue engineering.
The medical engineered materials market size is expected to see rapid growth in the next few years. It will grow to $43.79 billion in 2029 at a compound annual growth rate (CAGR) of 14.6%. The growth in the forecast period can be attributed to global health preparedness, 3D printing and additive manufacturing, advanced coatings, nanomedicine, personalized medicine. Major trends in the forecast period include nanotechnology, biodegradable and resorbable materials, bioactive and smart materials, sustainability and eco-friendly materials, advancements in medical technology.
The anticipated increase in chronic diseases is poised to propel the growth of the medical engineered materials market. Chronic diseases, characterized by lasting a year or more and requiring ongoing medical attention or limiting daily activities, are driving the demand for materials that can be engineered at the nano level. Nanoengineered components are utilized to construct miniature replicas of heart chambers, offering innovative solutions for treating heart-related issues. As indicated by the National Center for Biotechnology Information (NCBI) in January 2023, the global population with chronic diseases is projected to reach 142.66 million by 2050, a significant rise from 71.522 million in 2020. Hence, the escalating instances of chronic diseases serve as a catalyst for the growth of the medical engineered materials market.
The increasing number of surgical procedures is expected to be a driving force behind the growth of the medical engineered materials market. Surgical procedures involve medical interventions that necessitate incisions or invasive techniques to access and treat internal or external body areas. This surge in surgical procedures creates a heightened demand for materials used in surgical instruments, implantable devices, and minimally invasive procedures. The critical need for biocompatible and infection-resistant materials underscores their role in ensuring patient safety. Research and development driven by customization and regulatory standards, coupled with innovations in materials prompted by cost efficiency and technological advancements, contribute to the growth of the medical engineered materials market. For example, the Organization for Economic Co-operation and Development reported an 8.4% increase in cataract surgical procedures in the Czech Republic in October 2023, totaling 142,670 procedures in 2022 compared to 131,612 in 2021. Therefore, the rising number of surgical procedures acts as a growth driver for the medical engineered materials market.
Major players in the medical engineered materials market are strategically introducing new products to enhance their competitive position. An instance of this is Celanese Corporation, a US-based specialty materials company, launching Celanex MT PBT 2406MT GF20 in May 2022. This high-performance material is tailored for medical devices and various applications, offering exceptional strength, stiffness, and noise-free sliding capabilities. It finds applicability in Class I, Class II, and Class III medical device applications, as well as in automotive, electrical, electronics, industrial machinery, and consumer goods. The tribologically modified PBT+PET grade, reinforced with 20% glass fiber, provides versatile solutions across multiple industries.
Major companies in the medical engineered materials market are investing in the development of new products to gain a competitive edge. Covestro AG, a Germany-based polymer materials company, introduced Makrolon 3638 polycarbonate in February 2023. This advanced material solution, tailored for healthcare and life sciences applications, exhibits high durability across a wide temperature range, from cryogenic to steam sterilization conditions. It features high impact strength and ductility, along with high chemical resistance, meeting medical-grade and biocompatibility standards. Suitable for skin contact applications, the material is also of food contact quality and can be sterilized using common methods in the healthcare sector.
In February 2022, Celanese Corporation, a US-based global chemical leader, completed the acquisition of DuPont's resins and Advanced Solutions business lines for $11 billion. This strategic move is expected to position Celanese as a market leader with unparalleled scale, production capabilities, and technological expertise to cater to the automotive, consumer, and industrial markets. DuPont, a US-based company operating in medical engineered materials, is part of this acquisition.
Major companies operating in the medical engineered materials market include Evonik Industries AG, Covestro AG, BASF SE, Solvay SA, Saudi Basic Industries Corporation, Trelleborg AB, Koninklijke DSM N.V, Celanese Corporation, DuPont de Nemours Inc., Ethicon Inc., Henkel AG & Co. KGaA, Huntsman Corporation, Momentive Performance Materials Inc., Nitto Denko Corporation, Recticel Ltd., Victrex plc, Ensinger GmbH, RTP Company, Foster Corporation, Zeus Industrial Products Inc., Saint-Gobain SA, Arkema SA, Eastman Chemical Company, Lubrizol Corporation, PolyOne Corporation, Quadrant AG, Rochling Group, DSM Biomedical, Invibio Ltd., Carpenter Technology Corporation, Heraeus Medical Components GmbH, CeramTec GmbH, Morgan Advanced Materials plc, Freudenberg Medical LLC, Synectic Engineering Inc., Medtronic plc, Abbott Laboratories, Johnson & Johnson, Siemens Healthineers AG.
Asia-Pacific was the largest region in the medical engineered materials market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in this medical engineered materials market analysis report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa. The countries covered in the medical engineered materials market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA.
Medical engineered materials, often referred to as biomaterials, denote substances that are specifically crafted to meet particular or customized criteria, aiming to interact with biological systems for therapeutic or diagnostic purposes in the field of medicine. Serving as a crucial link between science, technology, and medicine, these materials are employed to integrate essential elements of materials science with a deep understanding of medical design.
The principal categories of medical engineered materials include medical plastics, medical foams, medical films, medical adhesives, and medical elastomers. Medical-grade plastic materials are formulated to produce items for medical applications. These materials find use in the manufacturing of products for in vitro diagnostics and primary packaging for pharmaceuticals, ensuring the preservation and containment of medicines to prevent contamination. The diverse applications span medical devices, medical disposables, medical wearables, and advanced wound care.
The medical engineered materials market research report is one of a series of new reports that provides medical engineered materials market statistics, including medical engineered materials industry global market size, regional shares, competitors with a medical engineered materials market share, detailed medical engineered materials market segments, market trends and opportunities, and any further data you may need to thrive in the medical engineered materials industry. This medical engineered materials market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The medical engineered materials market consists of sales of medical plastics, medical foams, medical films, medical elastomers, and medical adhesives. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Medical Engineered Materials Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on medical engineered materials market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for medical engineered materials? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The medical engineered materials market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) Covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Medical Plastics; Medical Foams; Medical Films; Medical Adhesives; Medical Elastomer2) By Application Type: Medical Devices; Medical Disposables; Medical Wearables; Advanced Woundcare
Subsegments:
1) By Medical Plastics: Polyethylene (PE); Polyvinyl Chloride (PVC); Polycarbonate (PC); Polypropylene (PP)2) By Medical Foams: Polyurethane Foams; Polyethylene Foams; Silicone Foams
3) By Medical Films: Thermoplastic Films; Biodegradable Films; Adhesive Films
4) By Medical Adhesives: Epoxy Adhesives; Cyanoacrylate Adhesives; Silicone Adhesives
5) By Medical Elastomers: Silicone Elastomers; Thermoplastic Elastomers (TPE); Polyurethane Elastomers
Key Companies Mentioned: Evonik Industries AG; Covestro AG; BASF SE; Solvay SA; Saudi Basic Industries Corporation
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Medical Engineered Materials market report include:- Evonik Industries AG
- Covestro AG
- BASF SE
- Solvay SA
- Saudi Basic Industries Corporation
- Trelleborg AB
- Koninklijke DSM N.V
- Celanese Corporation
- DuPont de Nemours Inc.
- Ethicon Inc.
- Henkel AG & Co. KGaA
- Huntsman Corporation
- Momentive Performance Materials Inc.
- Nitto Denko Corporation
- Recticel Ltd.
- Victrex plc
- Ensinger GmbH
- RTP Company
- Foster Corporation
- Zeus Industrial Products Inc.
- Saint-Gobain SA
- Arkema SA
- Eastman Chemical Company
- Lubrizol Corporation
- PolyOne Corporation
- Quadrant AG
- Rochling Group
- DSM Biomedical
- Invibio Ltd.
- Carpenter Technology Corporation
- Heraeus Medical Components GmbH
- CeramTec GmbH
- Morgan Advanced Materials plc
- Freudenberg Medical LLC
- Synectic Engineering Inc.
- Medtronic plc
- Abbott Laboratories
- Johnson & Johnson
- Siemens Healthineers AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 25.43 Billion |
| Forecasted Market Value ( USD | $ 43.79 Billion |
| Compound Annual Growth Rate | 14.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 40 |