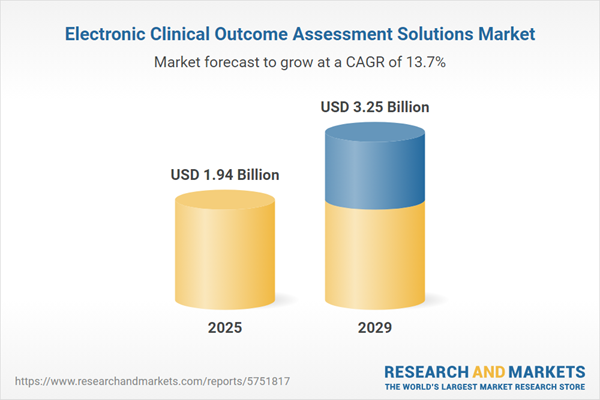
The electronic clinical outcome assessment solutions market size is expected to see rapid growth in the next few years. It will grow to $3.25 billion in 2029 at a compound annual growth rate (CAGR) of 13.7%. The growth in the forecast period can be attributed to integration with wearable devices, decentralized and hybrid trials, patient engagement strategies, security and compliance measures, remote monitoring and site collaboration. Major trends in the forecast period include artificial intelligence in clinical trials, clinical trial digitization, advancements in mobile technology, real-world evidence integration, patient-reported outcomes (PROs) emphasis.
The electronic clinical outcome assessment solutions market is anticipated to experience growth due to an upswing in research and development activities. Research and development activities encompass the initiatives undertaken by companies to innovate and introduce new products and services. Electronic clinical outcome assessment solutions contribute to these endeavors by electronically capturing outcomes data in clinical trials. As an illustration, in November 2023, the National Center for Science and Engineering Statistics, a US-based government agency, reported that in the fiscal year 2022, academic institutions allocated a total of $97.8 billion to research and development, indicating an increase of $8 billion compared to fiscal year 2021. Furthermore, internal funding for Research and Development (R&D) at universities exhibited a notable rise of $2.1 billion compared to the fiscal year 2021, while R&D funding from businesses to universities saw a growth of $587 million. Consequently, the surge in research and development activities is a key driver for the electronic clinical outcome assessment solutions market.
The anticipated growth of the electronic clinical outcome assessment solutions market is attributed to the rising number of clinical trials. Clinical trials are research studies or investigations conducted on human subjects to assess the safety, efficacy, and potential side effects of medical treatments, drugs, devices, or interventions. Electronic Clinical Outcome Assessment (eCOA) solutions play a vital role in clinical trials by offering a digital and streamlined approach to collecting patient-reported outcomes and other relevant data. For instance, as reported by the US-based digital media company Xtalks in May 2023, the global registration count on ClinicalTrials.gov reached 452,604 clinical trials in 2023, marking a significant increase from the reported over 365,000 registered trials in 2021. Furthermore, among these 452,604 clinical trials, 64,838 are actively recruiting participants. Hence, the escalating number of clinical trials serves as a catalyst for the growth of the electronic clinical outcome assessment solutions market.
Technological advancements are a significant trend gaining traction in the electronic clinical outcome assessment solutions market. Major companies in this sector are focused on developing innovative platforms to strengthen their market position. For example, in May 2024, EDETEK, a U.S.-based technology company specializing in digital solutions for the clinical trial sector, announced the launch of CONFORM eClinical Version 5.1. This comprehensive platform is designed to enhance and streamline clinical trial management processes. The new version integrates a wide array of components that facilitate efficient clinical research, addressing the evolving needs of sponsors and stakeholders within the pharmaceutical industry.
Key players in the electronic clinical outcome assessment solutions market are focusing their efforts on the development of innovative solutions, notably eCOA toolkits. An eCOA (Electronic Clinical Outcome Assessment) toolkit is a collection of software tools and resources strategically designed to facilitate the implementation and management of electronic methods for gathering patient-reported outcomes and other critical clinical trial data. As an illustration, in December 2022, Suvoda, a US-based software company, unveiled its electronic clinical outcome assessments (eCOA) design toolkit. This toolkit is seamlessly integrated with Suvoda IRT and eConsent, addressing previous shortcomings that have impacted the field of eCOA. It features a specialized language and authoring tool specifically crafted for the creation of eCOA questionnaires.
In November 2022, Citeline, a leading provider of pharmaceutical intelligence solutions based in the U.S., merged with Norstella in a deal valued at approximately $5 billion. This merger aims to enhance patient outcomes by improving access to therapies, streamlining drug development processes, and providing innovative solutions tailored to the evolving needs of the pharmaceutical industry. Norstella is recognized as a global leader in pharmaceutical intelligence and consulting services, also based in the U.S.
Major companies operating in the electronic clinical outcome assessment solutions market include IQVIA Holdings Inc., Oracle Corporation, ERT Clinical, Dassault Systemes SE, OmniComm Systems Inc., Parexel International Corporation, Veeva Systems Inc., KUKDO CHEMICAL CO. LTD., Medidata Solutions Inc., Signant Health, Clario Inc., ArisGlobal LLC, Merge Healthcare Inc., YPrime LLC, eClinical Solutions LLC, CRF Bracket Company, Anju Software Inc., Castor EDC B.V., Kayentis, Mednet Solutions LLC, Clinical Ink Inc., Cloudbyz Inc., Climedo Health GmbH, ClinCapture Inc.
North America was the largest region in the electronic clinical outcome assessment solutions market in 2024. Asia-Pacific is expected to be the fastest-growing region in the electronic clinical outcome assessment solutions market during the forecast period. The regions covered in the electronic clinical outcome assessment solutions market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the electronic clinical outcome assessment solutions market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Electronic clinical outcome assessment involves capturing outcome data electronically in clinical trials, streamlining the collection of crucial data points for analysis.
The primary categories of products within electronic clinical outcome assessment (eCOA) solutions include web-based eCOA solutions, on-premise-based eCOA solutions, and cloud-platform-based eCOA solutions. Web-based eCOA solutions denote programs distributed over a network, commonly a corporate intranet or the internet, and accessible through a standard web browser. These solutions encompass various approaches such as patient-reported outcome (PRO), clinician-reported outcome (ClinRO), observer-reported outcome (ObsRO), and performance outcome (PerfO). They find application in hospitals or healthcare providers, contract research organizations (CROs), pharmaceutical and biotechnology firms, medical device companies, and other end users.
The electronic clinical outcome assessment solutions market research report is one of a series of new reports that provides electronic clinical outcome assessment solutions market statistics, including electronic clinical outcome assessment solutions industry global market size, regional shares, competitors with an electronic clinical outcome assessment solutions market share, detailed electronic clinical outcome assessment solutions market segments, market trends and opportunities, and any further data you may need to thrive in the electronic clinical outcome assessment solutions industry. This electronic clinical outcome assessment solutions market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The electronic clinical outcome assessment solutions market consists of sales of eCOA live, eCOA multimedia, eCOA rescue studies, and suicidal ideation. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Electronic Clinical Outcome Assessment Solutions Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on electronic clinical outcome assessment solutions market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for electronic clinical outcome assessment solutions? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The electronic clinical outcome assessment solutions market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Web Based eCOA Solutions; on Premise Based eCOA Solutions; Cloud Platform Based eCOA Solutions2) By Approach: Patient-Reported Outcome (PRO); Clinician-Reported Outcome (ClinRO); Observer-Reported Outcome (ObsRO); Performance Outcome (PerfO)
3) By End-User: Hospitals or Healthcare Providers; Contract Research Organizations (CROs); Pharmaceutical and Biotechnology Firms; Medical Device Companies; Other End-Users
Subsegments:
1) By Web-Based eCOA Solutions: Patient-Reported Outcome (PRO) Tools; Clinician-Reported Outcome (ClinRO) Tools; Observer-Reported Outcome (ObsRO) Tools2) By on-Premise Based eCOA Solutions: Standalone Software Solutions; Integrated on-Premise Solutions
3) By Cloud Platform Based eCOA Solutions: Software As a Service (SaaS) eCOA Solutions; Mobile eCOA Applications; Hybrid Cloud Solutions
Key Companies Mentioned: IQVIA Holdings Inc.; Oracle Corporation; ERT Clinical; Dassault Systemes SE; OmniComm Systems Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- IQVIA Holdings Inc.
- Oracle Corporation
- ERT Clinical
- Dassault Systemes SE
- OmniComm Systems Inc.
- Parexel International Corporation
- Veeva Systems Inc.
- KUKDO CHEMICAL CO. LTD.
- Medidata Solutions Inc.
- Signant Health
- Clario Inc.
- ArisGlobal LLC
- Merge Healthcare Inc.
- YPrime LLC
- eClinical Solutions LLC
- CRF Bracket Company
- Anju Software Inc.
- Castor EDC B.V.
- Kayentis
- Mednet Solutions LLC
- Clinical Ink Inc.
- Cloudbyz Inc.
- Climedo Health GmbH
- ClinCapture Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.94 Billion |
| Forecasted Market Value ( USD | $ 3.25 Billion |
| Compound Annual Growth Rate | 13.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |