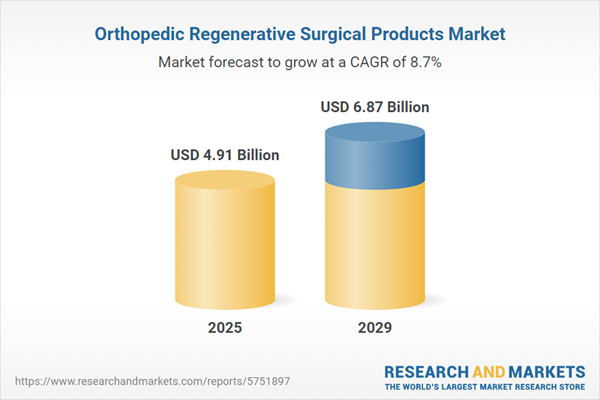
The orthopedic regenerative surgical products market size has grown strongly in recent years. It will grow from $4.54 billion in 2024 to $4.91 billion in 2025 at a compound annual growth rate (CAGR) of 8.3%. The growth in the historic period can be attributed to aging population, orthopedic injuries, patient demand, sports medicine, musculoskeletal disorders.
The orthopedic regenerative surgical products market size is expected to see strong growth in the next few years. It will grow to $6.87 billion in 2029 at a compound annual growth rate (CAGR) of 8.7%. The growth in the forecast period can be attributed to regulatory approval, sustainable practices, minimally invasive procedures, rehabilitation and physicalTherapy, joint preservation. Major trends in the forecast period include advanced biomaterials, surgical advancements, artificial intelligence (ai) and predictive analytics, regenerative medicine advancements, orthopedic implants.
The increasing prevalence of orthopedic diseases worldwide is expected to drive the growth of the orthopedic regenerative surgical products market. These products help prevent orthopedic issues such as fractures, sprains, and strains, as well as manage lifelong conditions that cause functional limitations and disabilities. For example, in June 2024, a report by the Australian Bureau of Statistics, an Australia-based statistical agency, revealed that musculoskeletal conditions accounted for 12.8% of the total disease burden (DALY), 23.1% of the non-fatal burden (YLD), and 0.8% of the fatal burden (YLL) in 2023. In 2022, these conditions were the underlying or associated cause of 10,446 deaths (40 per 100,000 population), representing 5.5% of all deaths. Therefore, the rising incidence of orthopedic diseases is driving the demand for orthopedic regenerative surgical products.
The anticipated growth of the orthopedic regenerative surgical products market is expected to be propelled by the increasing number of hospitals. Hospitals, as healthcare institutions, are essential in providing medical treatment, care, and services to individuals who are ill or injured. The adoption of orthopedic regenerative surgical products in hospitals is associated with enhanced patient outcomes, reduced risk of complications, faster healing and recovery, and the availability of advanced regenerative surgical options. As of May 2023, the American Hospital Association (AHA) reported an increase in the total number of hospitals to 6,129 compared to 6,093 in 2022, with total admissions across all U.S. hospitals reaching 34,011,386 compared to 33,356,853 in the previous year. Therefore, the growing number of hospitals is a significant factor driving the expansion of the orthopedic regenerative surgical products market.
Technological advancements are emerging as a key trend in the orthopedic regenerative surgical products market. For example, in May 2022, Ocugen Inc., a U.S.-based biopharmaceutical company, introduced NeoCart, a Phase 3 cell therapy platform technology (autologous chondrocyte-derived neocartilage). NeoCart recently received Regenerative Medicine Advanced Therapy (RMAT) designation from the U.S. Food and Drug Administration (FDA) for the treatment of full-thickness lesions in adult knee cartilage.
Major companies in the orthopedic regenerative surgical products market are strategically focusing on innovative products like the non-permanent ossiofiber compression staple to drive market revenues. This medical device is utilized in orthopedic and musculoskeletal surgeries for fracture fixation and bone fusion, providing temporary stability and compression to support healing and fusion. For instance, Ossio Inc., a US-based company specializing in orthopedic and musculoskeletal applications, launched the non-permanent ossiofiber compression staple in February 2023. Combining mechanical strength and spontaneous bone regeneration, this staple offers an alternative to traditional implants, such as resorbable and allograft options, as well as permanent metal hardware. Ossiofiber Compression Staples provide solutions for various midfoot and hindfoot procedures, including flatfoot corrective procedures, Lapidus fusions, and midfoot fusions.
In April 2022, Isto Biologics, a US-based company specializing in osteobiologic regeneration technologies and cell-based therapeutics, acquired TheraCell Inc. for an undisclosed sum. The acquisition aims to strengthen Isto Biologics' portfolio of surgical and clinical care solutions in the areas of spine, orthopedics, and sports medicine. TheraCell, Inc., a U.S.-based regenerative medicine company, focuses on developing innovative products for bone repair and tissue regeneration.
Major companies operating in the orthopedic regenerative surgical products market include Baxter International Inc., Zimmer Biomet Holdings Inc., Stryker Corporation, Smith&Nephew plc, Allosource, Anika Therapeutics Inc., Vericel Corporation, Amniox Medical Inc., MIMEDX Group Inc., Aptissen S.A, Arthrex Inc., Bioventus LLC, Conmed Corporation, DePuy Synthes Inc., Exactech Inc., Globus Medical Inc., Integra LifeSciences Corporation, Johnson & Johnson Medical Devices group, Kuros Biosciences AG, Medtronic plc, NuVasive Inc., Orthofix Medical Inc., Osiris Therapeutics Inc., RTI Surgical Inc., SeaSpine Holdings Corporation, Terumo BCT Inc., Tissue Regenix Group plc, Wright Medical Group N.V., Xtant Medical Holdings Inc., Camber Spine Technologies, Lima Corporate, Life Spine Inc., CollPlant Biotechnologies Ltd.
North America was the largest region in the orthopedic regenerative surgical products market in 2024 and is also expected to be the fastest-growing region in the forecast period. The regions covered in orthopedic regenerative surgical products market analysis report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, and Africa. The countries covered in the orthopedic regenerative surgical products market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA.
Orthopedic regenerative surgical products play a crucial role in treating various musculoskeletal conditions, including joint pains, gout, articular defects, fibromyalgia, osteoarthritis, trauma, joint replacement, and other orthopedic injuries. These products are particularly significant in regenerative orthopedic medicine, where nonsurgical treatments like platelet-rich plasma (PRP) are often chosen to aid in the healing and repair of musculoskeletal injuries, such as those affecting joints, tendons, ligaments, and muscles.
The main types of orthopedic regenerative surgical products include allografts, synthetic materials, cell-based products, and viscosupplements. Allografts involve the transplantation of tissue between genetically distinct individuals of the same species. Examples of human allografts include anterior tibialis tendon, frozen femoral head, freeze-dried bone chips, demineralized bone matrix (DBM) putty, acellular dermis, and amniotic membrane. These regenerative products are utilized by hospitals, ambulatory surgical centers, and other healthcare facilities for the treatment of orthopedic conditions such as pain management, trauma repair, cartilage and tendon repair, and joint reconstruction.
The orthopedic regenerative surgical products market research report is one of a series of new reports that provides orthopedic regenerative surgical products market statistics, including orthopedic regenerative surgical products industry global market size, regional shares, competitors with an orthopedic regenerative surgical products market share, detailed orthopedic regenerative surgical products market segments, market trends and opportunities, and any further data you may need to thrive in the orthopedic regenerative surgical products industry. This orthopedic regenerative surgical products market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The orthopedic regenerative surgical products market consists of sales of allograft wedges, allowrap DS, BIO⁴, DBM, hydroset, imbibe, nerve repair, and prochondrix CR.Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Orthopedic Regenerative Surgical Products Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on orthopedic regenerative surgical products market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for orthopedic regenerative surgical products? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The orthopedic regenerative surgical products market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) by Product: Allograft, Synthetic, Cell-based, Viscosupplements2) by Application: Orthopedic Pain Management, Trauma Repair, Cartilage and Tendon Repair, Joint Reconstruction
3) by End User: Hospitals, Ambulatory Surgical Centers, Other End Users
Subsegments:
1) by Allograft: Bone Allograft; Soft Tissue Allograft; Osteoinductive Allograft; Osteoconductive Allograft2) by Synthetic: Synthetic Bone Grafts; Synthetic Soft Tissue Grafts; Composite Synthetic Grafts
3) by Cell-based: Stem Cell Therapies; Platelet-Rich Plasma (PRP); Mesenchymal Stem Cells (MSCs)
4) by Viscosupplements: Hyaluronic Acid Injections; Sodium Hyaluronate; Other Viscosupplement Products
Key Companies Mentioned: Baxter International Inc.; Zimmer Biomet Holdings Inc.; Stryker Corporation; Smith&Nephew plc; Allosource
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Orthopedic Regenerative Surgical Products market report include:- Baxter International Inc.
- Zimmer Biomet Holdings Inc.
- Stryker Corporation
- Smith&Nephew plc
- Allosource
- Anika Therapeutics Inc.
- Vericel Corporation
- Amniox Medical Inc.
- MIMEDX Group Inc.
- Aptissen S.A
- Arthrex Inc.
- Bioventus LLC
- Conmed Corporation
- DePuy Synthes Inc.
- Exactech Inc.
- Globus Medical Inc.
- Integra LifeSciences Corporation
- Johnson & Johnson Medical Devices group
- Kuros Biosciences AG
- Medtronic plc
- NuVasive Inc.
- Orthofix Medical Inc.
- Osiris Therapeutics Inc.
- RTI Surgical Inc.
- SeaSpine Holdings Corporation
- Terumo BCT Inc.
- Tissue Regenix Group plc
- Wright Medical Group N.V.
- Xtant Medical Holdings Inc.
- Camber Spine Technologies
- Lima Corporate
- Life Spine Inc.
- CollPlant Biotechnologies Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 4.91 Billion |
| Forecasted Market Value ( USD | $ 6.87 Billion |
| Compound Annual Growth Rate | 8.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 33 |