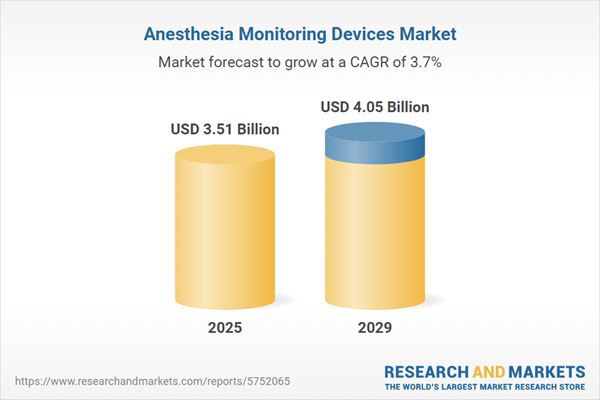
The anesthesia monitoring devices market size has declined in recent years. It will decline from $3.67 billion in 2024 to $3.51 billion in 2025 at a compound annual growth rate (CAGR) of -4.5%. The decline in the historic period can be attributed to high cost of anesthesia monitoring devices, high cost of maintenance, covid Impact.
The anesthesia monitoring devices market size is expected to see steady growth in the next few years. It will grow to $4.05 billion in 2029 at a compound annual growth rate (CAGR) of 3.7%. The growth in the forecast period can be attributed to growing healthcare expenditure, remote monitoring and telemedicine, rise in minimally invasive surgery, rise in economic growth. Major trends in the forecast period include integration with electronic health records (EHR), artificial intelligence (AI) and machine learning, mobile apps and wearables, advanced sensors and connectivity, non-invasive monitoring technologies.
The global rise in the number of surgeries is projected to drive the growth of the anesthesia monitoring devices market. As the frequency of surgeries such as hip fracture repairs, neurosurgical procedures, cesarean sections, and others increases, there is a corresponding surge in demand for anesthesia monitoring devices. For instance, in June 2024, the International Society of Aesthetic Plastic Surgery (ISAPS), a US-based organization of certified aesthetic plastic surgeons, reported that the total number of surgical and non-surgical procedures increased by 3.4% in 2023, reaching 34.9 million. Consequently, the growing volume of surgeries is expected to boost the anesthesia monitoring devices market.
The growing geriatric population is expected to significantly enhance the growth of the anesthesia monitoring device market in the coming years. The term "geriatric population" refers to elderly individuals or senior citizens within a specific society or demographic. As the number of older adults increases, there is a corresponding rise in surgical procedures, which necessitates careful anesthetic management and the use of anesthesia monitoring devices to ensure patient safety. For example, data released by the House of Commons in July 2024 indicated that in 2022, around 12.7 million people aged 65 and older lived in the UK, accounting for 19% of the total population. Thus, the increasing geriatric population is a key factor driving the growth of the anesthesia monitoring device market.
Technological advancements represent a prominent trend gaining traction within the anesthesia monitoring devices market. Numerous companies are introducing innovative, technologically advanced products in this sector. For instance, in April 2022, SCHILLER, a Switzerland-based company, launched the MRI-compatible patient monitor MAGLIFE RT-1. This state-of-the-art system is designed for patient monitoring in an MRI environment, ensuring comprehensive tracking of vital parameters during anesthesia. The MAGLIFE RT-1 facilitates close monitoring during examinations and can be entirely controlled from outside the Faraday cage through the MAGSCREEN RT-1. This system is versatile, catering to patients across different age groups, encompassing adults, children, and neonates.
Prominent players in the anesthesia monitoring device market are increasingly embracing strategic collaborations as a key aspect of their business strategies. These strategic partnerships entail cooperative relationships formed between two or more organizations with the specific aim of achieving defined business objectives and goals. An illustration of this can be observed in May 2023, when Senzime AB, a Sweden-based company specializing in the production of patient monitoring systems, announced an expansion of its collaboration with Fukuda Denshi, a renowned Japanese-based company with expertise in patient monitoring. This extended partnership encompasses the manufacturing of TetraGraph systems, a cutting-edge technology developed by Senzime. The TetraGraph technology is instrumental in monitoring neuromuscular blockade during anesthesia and is founded on electromyography (EMG) technology, offering precise and sensitive readings.
In May 2023, SunMed, a US-based manufacturer of consumable medical devices tailored for anesthesia and respiratory care, executed the acquisition of Vyaire Medical for an undisclosed sum. This strategic move positions SunMed as a leading manufacturer solely dedicated to airway management and operative care, providing a more comprehensive range of offerings for healthcare practitioners. The acquisition solidifies SunMed's role as a one-stop source for high-quality consumable respiratory and anesthesia medical products, thereby enhancing the support for optimal patient outcomes. Vyaire Medical, a US-based medical device company specializing in anesthesia care products and solutions, is a key component of this strategic initiative.
Anesthesia monitoring devices play a crucial role in evaluating a patient's responsiveness to anesthetic drugs during surgical procedures. These instruments aid anesthetists in monitoring and displaying the precise dosage of anesthetic drugs administered to the patient.
The market offers diverse product types of anesthesia monitoring devices, including advanced anesthesia monitors, basic anesthesia monitors, and integrated anesthesia workstations. Advanced options encompass anesthesia gas monitors, depth of anesthesia monitors, and standalone capnography. Basic monitors cater to essential measurements such as oxygenation, ventilation, circulation, and body temperature. Anesthesia monitoring devices find applications across various medical specialties, including cardiology, neurology, dental, ophthalmology, urology, orthopedics, and more. End users of anesthesia monitoring devices span across hospitals, ambulatory surgical centers, clinics, and nursing homes. These devices are pivotal in ensuring optimal patient care and safety during medical procedures.
The anesthesia monitoring devices market research report is one of a series of new reports that provides anesthesia monitoring devices market statistics, including anesthesia monitoring devices industry global market size, regional shares, competitors with anesthesia monitoring devices market share, detailed anesthesia monitoring devices market segments, market trends and opportunities, and any further data you may need to thrive in the anesthesia monitoring devices industry. This anesthesia monitoring devices market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
Major companies operating in the anesthesia monitoring devices market include Baxter International Inc., Cardinal Health Inc., Draegerwerk AG & Co. KGaA, GE Healthcare, Heyer Medical AG, Intersurgical Limited, Medtronic Inc., Mindray Medical International Limited, Nihon Kohden Corporation, Philips Healthcare, Smiths Medical Inc., Infinium Medical Inc., Fukuda Denshi Co. Ltd., Becton Dickinson and Company, Ambu A/S, Teleflex Incorporated, Ventlab, ACOMA Medical Industry Co., BPL Medical Technologies, Dixion GmbH, Löwenstein Medical GmbH & Co. KG, Medasense Biometrics Ltd., Veterinary Anesthesia Systems Inc., CNSystems Medizintechnik GmbH, Allied Medical Limited, Criticare Technologies Inc., Masimo Corporation, Spacelabs Healthcare Inc., Mennen Medical Ltd., BrainU Co. Ltd., OSI Systems Inc., Penlon Limited, Natus Medical Incorporated, Schiller AG, Edwards Lifesciences Corporation, Bionet Co. Ltd., DRE Medical Inc., Digicare Biomedical Technology Inc.
North America was the largest region in the anesthesia monitoring devices market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the anesthesia monitoring devices market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, and Africa. The countries covered in the anesthesia monitoring devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA.
The anesthesia monitoring devices market consists of sales of clinician monitoring, Respiratory monitoring system, Circulatory system monitoring, and temperature monitoring system. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Anesthesia Monitoring Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on anesthesia monitoring devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for anesthesia monitoring devices ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The anesthesia monitoring devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Advanced Anesthesia Monitors; Basic Anesthesia Monitors; Integrated Anesthesia Workstation2) By Application: Cardiology; Neurology; Dental; Ophthalmology; Urology; Orthopedics; Other Applications
3) By End User: Hospitals; Ambulatory Surgical Centers; Clinics; Nursing Homes
Subsegments:
1) By Advanced Anesthesia Monitors: Multi-parameter Monitors; EEG Monitors; Gas Analyzers2) By Basic Anesthesia Monitors: Pulse Oximeters; Blood Pressure Monitors; Capnometers
3) By Integrated Anesthesia Workstation: Anesthesia Delivery Systems; Ventilation Systems; Monitoring Systems
Key Companies Mentioned: Baxter International Inc.; Cardinal Health Inc.; Draegerwerk AG & Co. KGaA; GE Healthcare; Heyer Medical AG
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Anesthesia Monitoring Devices market report include:- Baxter International Inc.
- Cardinal Health Inc.
- Draegerwerk AG & Co. KGaA
- GE Healthcare
- Heyer Medical AG
- Intersurgical Limited
- Medtronic Inc.
- Mindray Medical International Limited
- Nihon Kohden Corporation
- Philips Healthcare
- Smiths Medical Inc.
- Infinium Medical Inc.
- Fukuda Denshi Co. Ltd.
- Becton Dickinson and Company
- Ambu A/S
- Teleflex Incorporated
- Ventlab
- ACOMA Medical Industry Co.
- BPL Medical Technologies
- Dixion GmbH
- Löwenstein Medical GmbH & Co. KG
- Medasense Biometrics Ltd.
- Veterinary Anesthesia Systems Inc.
- CNSystems Medizintechnik GmbH
- Allied Medical Limited
- Criticare Technologies Inc.
- Masimo Corporation
- Spacelabs Healthcare Inc.
- Mennen Medical Ltd.
- BrainU Co. Ltd.
- OSI Systems Inc.
- Penlon Limited
- Natus Medical Incorporated
- Schiller AG
- Edwards Lifesciences Corporation
- Bionet Co. Ltd.
- DRE Medical Inc.
- Digicare Biomedical Technology Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 175 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.51 Billion |
| Forecasted Market Value ( USD | $ 4.05 Billion |
| Compound Annual Growth Rate | 3.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 39 |