In its preliminary phase, the COVID-19 pandemic significantly impacted the high throughput process development (HTPD) market due to disruptions in research and development activities worldwide. As the pandemic continued worsening, it accelerated the vaccine and drug development process against the SARS-CoV-2 virus. As per the November 2022 update by BGI Genomics, an integrated solutions provider of precision medicine with the use of HTPD, upon the onset of the pandemic, the Institute of Molecular Genetic and Genetic Engineering (IMGGE) at the University of Belgrade immediately announced offering its expertise in molecular diagnostic methods. A partnership between BGI and IMGGE commenced with the construction of two permanent Huoyan laboratories. The first laboratory was established on the premises of the University Clinical Center of Serbia approximately 40 days after the pandemic was publicly disclosed. One of the two national laboratories for molecular diagnostics of infectious agents was established in Belgrade. By July 2020, the second National Laboratory, strategically positioned in the Serbian city of Nis to serve the region, was operational. Such developments contributed to the market's growth amid the pandemic.
In the post-pandemic phase, the market is expected to witness significant growth. For instance, as per the article published in September 2022 by Frontiers in Medical Technology, automated microfluidic technologies have considerable assurance for improving COVID-19 diagnostic test accessibility and speeding up high throughput detection techniques, specifically for POC testing. Additionally, COVID-19 diagnosis can easily be performed using the automated microfluidic platforms created for other pathogens. Thus, per the analysis, with the anticipated emergence of newer SARS-CoV-2 strains globally, the market is predicted to gain traction over the coming years.
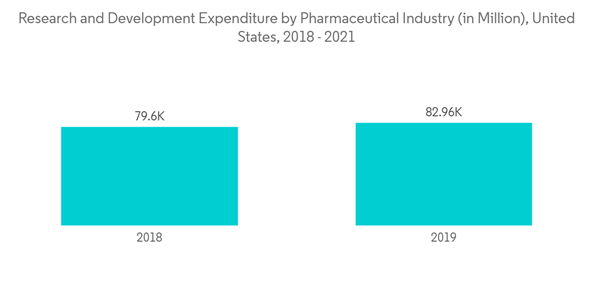
Additionally, the growing need for identifying new drug targets, advancements in drug discovery and development technology, an increase in pharmaceutical research and development expenditure, and the benefits associated with HTPD in terms of cost reduction and process efficiency are major factors driving market growth in the coming years.
With high throughput screening, numerous samples can be examined simultaneously under the same circumstances. In addition, with robotic capabilities, hundreds and thousands of samples could be examined at once. The high throughput process development is speedier and more affordable for large sample sizes, making it a convenient option for screening newer drug targets for drug development. Such advantages offered by high throughput process development in the drug discovery process, where several potential drug candidates need to be screened, are expected to boost the market's growth shortly.
Moreover, a million human genomes were sequenced in 2020 due to high throughput sequencing technologies, revolutionizing genetics and genomics. As a result, the pharmaceutical industry now has access to a wide range of prospective therapeutic targets that demand additional investigation, driving the demand for high throughput process development.
Likewise, growing research and development in the pharmaceutical industry will contribute to the market's growth. For clinical trials of neurodegenerative diseases like Alzheimer's, drugs that target the central nervous system (CNS) have a historically high failure rate. Also, time consumption and costliness of in-vivo screening remain essential components of preclinical testing. High throughput technologies have recently made it possible for these tests better to meet the demands of the current pharmacological development pace. For instance, in June 2021, scientists from the University of Artois in France miniaturized a patented BBB in-vitro model, enabling automated high throughput compound screening. Robotic cell seeding, assay testing, and imaging were used to automate the model, which had previously been carried out manually using a 12-well format. Automation improved accuracy and decreased the time needed for assay execution by researchers. It significantly increased precision and reduced the time spent by researchers performing assays. Ultimately, this allowed more compounds to be screened in the early stages of drug discovery.
Furthermore, new product launches are contributing to the market's growth. For instance, in April 2021, Thermo Fisher Scientific received USFDA approval for high throughput and integrated COVID-19 Test Kit. Likewise, in November 2022, the FDA granted a EUA for Roche's COBAS MPXV high throughput test to detect mpox. Patients could immediately achieve the desired results with this high throughput solution in order to receive the right care as quickly as possible without being compelled to undergo needless additional testing or isolation. In December 2021, Becton, Dickinson, and Company's COR System was enhanced by adding a new MX instrument for high throughput molecular testing for infectious diseases.
Therefore, the studied market is expected to witness significant growth over the forecast period owing to the above-mentioned factors. However, the high cost of technology and the lack of infrastructure may impede the market's growth.
High Throughput Process Development Market Trends
Biopharmaceutical & Biotechnology Companies Segment Expected to Witness Significant Growth
The biopharmaceutical & biotechnology companies segment by the end-user is expected to witness significant growth over the analysis period. This is primarily attributed to the increase in R&D activities and spending by the companies and the emerging need to identify new drug targets for drug discovery.Furthermore, significant factors augmenting the segment growth are a surge in the adoption of high throughput process development in these companies, the introduction of newer and advanced platforms, technological advancements in the fields of drug development, and growth in strategic developments by key players within the market. For instance, in November 2022, Mission Bio Inc., a pioneer in high-throughput single-cell DNA and multi-omics analysis, stated that AVROBIO's pipeline for lysosomal disorders deployed one single-cell transduction assay. Using Mission Bio's Tapestri Platform, the two firms worked together to create a precise, reproducible assay for measuring the proportion of transduced cells in samples made up of thousands of cells. At the 4th Annual Gene Therapy Analytical Development (GTAD) Summit, AVROBIO presented results on using this test to estimate the percentage transduction in autologous, lentiviral vector-mediated, genetically modified drug products.
Likewise, in June 2022, the United Kingdom's innovation agency, Innovate UK, gave a Smart grant to Orbit Discovery Ltd. This privately held biopharmaceutical firm specializes in identifying candidate peptide therapies using unique affinity and cell-based functional screening platforms. The funding, which has a total value of GBP 472,000 (~USD 579,474), will make it easier to use droplet-based microfluidics for cell-based functional screening and considerably increase the capacity and throughput of Orbit's peptide display platform. The high-throughput screening platform will target multi-membrane-spanning proteins such as G-protein-coupled receptors as difficult-to-access therapeutic targets in important biologically relevant areas.
In July 2022, Porvair Sciences, a manufacturer and developer of cutting-edge porous plastic materials and microplate technologies for the biotechnology and life science industries, introduced eGecko. This automated barcode application system offers the best high-throughput solution for accurately applying barcode labels onto racks of plates and petri dishes.
Such developments are contributing to the biopharmaceutical and biotechnology companies' growth over the forecast period.
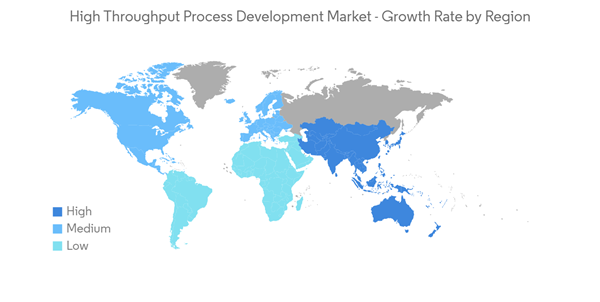
North America Expected to Witness Significant Growth
North America is expected to dominate the market owing to various factors, such as the easy availability of technologically advanced products, the growth in the prevalence of chronic diseases, higher spending on research and development activities, the presence of prominent players in the region, and well-established healthcare infrastructure.According to the article published by the Canadian Cancer Society in May 2022, an estimated 3,200 Canadians will be diagnosed with brain and spinal cord cancer by the end of 2022, including 1,850 men and 1,350 women. In addition, as per the source above, 27 Canadians are diagnosed with brain tumors every day. It is estimated that 55,000 Canadians are surviving with brain tumors. The high prevalence of brain tumors among the target population underlines the need for innovative therapies. This drives demand for new drug discovery and development, in turn boosting the adoption of high throughput process development among biopharmaceutical companies.
Furthermore, strategic initiatives by the regional companies augment the market’s growth. For instance, in September 2022, Arsenal Biosciences, Inc. collaborated with Genentech. Genentech will deploy ArsenalBio’s proprietary technology for high throughput process development and engineering of T-cells to identify critical success circuits in T-cell-based therapies. This discovery process employs the convergence of automation, large-scale genome engineering, and artificial intelligence algorithms to aid and advance the design, building, and testing of next-generation cell therapies for cancer.
In addition, Dynegene Technologies, with the first DNA high throughput synthesis expertise in China, was featured at the 2nd American Annual Conference of Human Genetics (ASHG). The company stated that the high throughput DNA synthesis technology of Dynegene Technologies would enter the United States market in the future to deliver overseas users high-quality, cost-effective products and services. It will provide robust support for synthetic biology, biomedical tools, molecular diagnostic materials, and other fields. Such developments are meant to speed up the use of high throughput process development across several end-users. Thus, owing to such developments, the North American region is expected to witness significant growth over the forecast period.
High Throughput Process Development Market Competitor Analysis
The high throughput process development market is slightly consolidated and competitive due to the presence of companies operating globally and regionally. The competitive landscape includes an analysis of a few international as well as local companies that hold market shares and are well known, such as Danaher Corporation, General Electric Company (GE Healthcare), Thermo Fisher Scientific Inc., Merck Millipore Limited, Agilent Technologies, Inc., Bio-Rad Laboratories, Inc., Eppendorf SE, Perkinelmer, Inc., Sartorius Stedim Biotech GmbH, and Tecan Group AG.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Danaher Corporation
- General Electric Company (GE Healthcare)
- Thermo Fisher Scientific Inc.
- Merck
- Agilent Technologies, Inc.
- Bio-Rad Laboratories, Inc.
- Eppendorf SE
- Perkinelmer, Inc.
- Sartorius Stedim Biotech GmbH
- Tecan Group AG