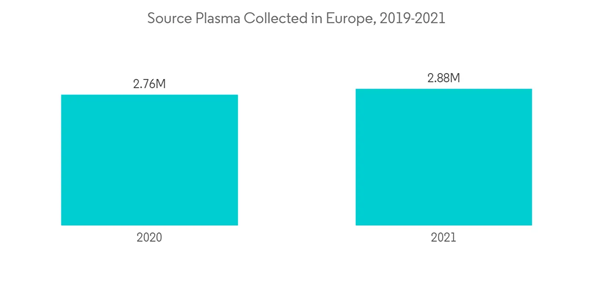
The COVID-19 pandemic has significantly impacted the blood transfusion diagnostics market throughout the year. Strict regulation, social distancing, home quarantine, and other restrictions significantly impacted the blood donation and blood collection procedures. This resulted in a decreased amount of blood transfusion. For instance, according to American Red Cross, in March 2020, it was reported that a severe blood crisis was there owing to a huge number of blood donation drive cancellations for the COVID-19 outbreak which resulted in 86,000 fewer blood donations. Also, according to a research article by NCBI in March 2022, it was reported that active and inactive donors both were reluctant to donate blood which significantly impacted the overall blood donation intention. This alternatively decreased the need for transfusion diagnostic and hindered the adoption of transfusion diagnostic kits and other instruments. Apart from that, owing to relocating medical staff and lab technicians to the COVID-19 department, there was a shortage of healthcare professionals for blood transfusion diagnostics during the pandemic. This resulted in the slowdown of blood transfusion diagnostics procedures. However, in the post-pandemic situation, a thrive was observed for blood donation to diminish the effect of the pandemic. This alternatively offsets the impact of blood shortage and normalizes the situation for blood transfusion.
Further, the increasing prevalence of blood-related disorders, including hemophilia, thrombocytopenia, and other disorders, is increasing the demand for blood transfusion. This, in turn, is increasing the demand and adoption of blood transfusion diagnostics. For instance, according to an article published by Medscape on March 2022, it was reported that the prevalence of Hemophilia A is 20.6 cases per 100,000 males in the United States. Also, according to an article published by the Canadian Hemophilia Society (CHS) in February 2022, it was reported that the total number of Canadian suffering from Hemophilia A and B is estimated to be 3,100. Thus, the rising prevalence of blood-related disorders propels the demand and adoption of blood transfusion diagnostic products during the study period.
Moreover, the rising initiatives by the for-profit and not-for-profit organizations to increase blood donation awareness positively impact the market growth. For instance, according to a press release by the U.S. Department of Health & Human Services on August 2022, it was reported that the U.S. Department of Health and Human Services (HHS) announced a campaign to increase awareness of donating blood and plasma. Also, in October 2022, NHS Blood and Transplant introduced a sickle cell blood donation campaign to increase the number of blood donors for sickle cell anemia. Thus, an increasing number of awareness campaigns on blood donation and transfusion is alternatively increasing the demand for blood transfusion diagnostic products and fueling the market growth.
Therefore, due to the above factors, the market is anticipated to grow substantially during the study period. However, certain factors, including adverse reactions during the blood transfusion procedure, limit the market's growth to some extent.
Key Market Trends
Reagents & Kits Segment is Anticipated to Hold a Significant Share Over the Forecast Period
Based on product types, the reagents & kits segment is expected to contribute significantly to the Blood Transfusion Diagnostics Market during the study period. The dominance is attributed to the repeated use of reagents & kits during the transfusion diagnostics procedure both for the donor and recipient. Also, lower shelf life as compared to the instruments is the other factor for the repetitive use of these consumables during transfusion diagnostic procedures.Apart from that, the comparatively lower price of the reagents & kits consumables is surging the demand and adoption of these products among healthcare professionals for blood transfusion diagnostics. For instance, according to an article published by Nexstar Media Inc. in August 2022, it was reported that the average selling price of a blood type test kit is estimated a USD 10. In contrast, the average selling price of the instruments is much higher than the reagent and kits.
Moreover, introducing affordable reagents and kits by the major players is fueling the adoption rate among the common people. For instance, in November 2022, Grifols, S.A., announced the Food and Drug Administration (FDA) approval of an AlphaID screening kit to detect alpha-1-antitrypsin deficiency in the comfort of a home. This helped the United States population to detect any genetic risks associated with alpha-1. Also, in July 2022, Immucor Inc. received FDA approval for Blood Grouping Reagents (monoclonal), Anti-Jka, Anti-Jkb, Anti-S, Anti-s, and Anti-Fya (IgG).
Therefore, owing to the above-mentioned factors, the consumables segment is anticipated to witness a significant share over the forecast period.
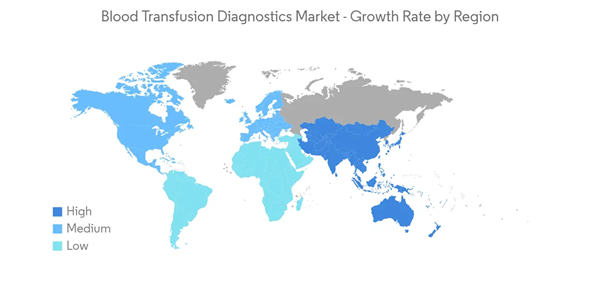
North America is Expected to Contribute a Significant Proportion to the Market
North America is expected to dominate the market owing to factors such as high awareness of blood transfusion along with the availability of advanced healthcare facilities for transfusion diagnostics. For instance, according to a report by America Red Cross, an estimated 16 million blood components are transfused each year in the United States. This significant amount of blood transfusion requires prior diagnostics and screening. This, in turn, increases the demand for blood transfusion diagnostics instruments and consumables during the study period.Apart from that, the rising prevalence of key disorders, including anemia, hemophilia, thalassemia, and others, is fostering the demand for blood transfusion. For instance, according to data by the Center for Disease Control and Prevention (CDC) published in May 2022, it was reported that an estimated 100,000 population in the United States were affected by sickle cell anemia. Also, according to an article published by the Journal of Public Health and Emergency in September 2022, it was reported that the prevalence of anemia was around 24% in Mexico. This huge patient population is fueling the demand for blood transfusion as well as transfusion diagnostics products during the study period.
Thus, due to the above factors, the North America region is contributing a significant share of the global blood transfusion diagnostics market.
Competitive Landscape
The Blood Transfusion Diagnostics Market is consolidated in nature. The competitive landscape includes an analysis of a few international and local companies that hold market shares and are well known. The key players in the market include Grifols, S.A., F. Hoffmann-La Roche Ltd., Immucor, Inc., Bio-Rad Laboratories, Inc., Quotient Suisse SA, Merck KGaA, Abbott, Diasorin S.p.A, Danaher, and Werfen among others.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Grifols, S.A.
- Immucor, Inc.
- Bio-Rad Laboratories, Inc.
- F. Hoffmann-La Roche Ltd
- QUOTIENT
- Merck KGaA
- Abbott
- Diasorin S.p.A
- Danaher
- Werfen