Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
These vaccines stand out for their enhanced safety profile, shorter development timelines, and adaptability across a broad spectrum of diseases. Positioned within the dynamic vaccine industry, the DNA vaccine market is poised for rapid evolution driven by continuous technological breakthroughs, expanded clinical applications, and heightened global health priorities. Companies that align with regulatory trends and innovate in delivery technologies are expected to gain significant competitive advantages.
Key Market Drivers
Rising Prevalence of Infectious and Chronic Diseases
The escalating occurrence of both infectious and chronic diseases is a critical driver for the Global Human DNA Vaccine Market. In 2021, approximately 18 million people died prematurely from noncommunicable diseases (NCDs), with 82% of these deaths occurring in low- and middle-income countries (LMICs), underscoring the disproportionate impact on economically disadvantaged regions.Furthermore, LMICs accounted for 73% of all NCD-related deaths globally. This significant health burden amplifies the need for innovative vaccination approaches like DNA vaccines, which are gaining momentum due to their unique benefits. Notably, in 2023, about 1.3 million people contracted HIV worldwide, a 39% reduction in new cases from 2010, indicating a global infection rate of 0.17 per 1,000 uninfected individuals. As diseases such as HIV, Hepatitis, Tuberculosis, HPV, COVID-19, Zika, and Ebola continue to challenge public health systems, DNA vaccines offer scalable, adaptable, and long-lasting immune protection - traits that are increasingly in demand globally.
Key Market Challenges
Limited Clinical Success and Regulatory Approvals
Despite encouraging outcomes in preclinical stages, human DNA vaccines face challenges in achieving strong clinical efficacy. A primary obstacle is their lower immunogenicity in humans when compared to traditional vaccine types, which often leads to suboptimal immune responses in clinical settings - especially when conventional administration methods are used. Consequently, regulatory approval for human use remains limited, with most authorizations to date focused on veterinary applications. Although the pandemic has accelerated interest in DNA vaccines for humans, regulatory bodies continue to require comprehensive data on long-term safety and efficacy. These stringent and often uncertain regulatory demands present a substantial hurdle, particularly for smaller biotech firms, thereby slowing commercial progress and investment in the field.Key Market Trends
Shift Toward Platform-Based Vaccine Technologies
The vaccine sector is witnessing a strong trend toward platform-based technologies, which leverage a unified system for developing multiple vaccines. DNA vaccine platforms exemplify this model. By standardizing delivery mechanisms, production processes, and clinical trial protocols, these platforms significantly cut down on development time and costs. They also enable rapid responses to emergent health threats, supporting quicker vaccine rollouts. Pharmaceutical companies and investors are increasingly attracted to this scalable framework, which not only fosters efficient product pipelines but also strengthens pandemic preparedness strategies. These systems support diverse disease applications, enhance public-private collaborations, and draw funding from global health stakeholders.Key Market Players
- BOEHRINGER INGELHEIM GmbH (Merial)
- ELI-LILLY (Novartis Animal Health)
- Gene One Life Science
- GEOVAX LABS, INC
- Inovio Pharmaceuticals (VGX Animal Health)
- Genexine, Inc.
- VIATRIS INC. (Rottapharm Biotech)
- Takara Holdings (Takara Bio)
- ZOETIS INC. (Fort dodge Animal Health)
- Zydus Lifesciences Limited
Report Scope:
In this report, the Global Human DNA Vaccine Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Human DNA Vaccine Market, By Route of Administration:
- Intramuscular
- Subcutaneous
- Intradermal
- Others
Human DNA Vaccine Market, By Application:
- Oncology
- Tuberculosis
- HIV
- Others
Human DNA Vaccine Market, By End User:
- Hospitals & Clinics
- Biotechnology & Pharmaceutical Companies
- Academic & Research Institutions
- Others
Human DNA Vaccine Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Human DNA Vaccine Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- BOEHRINGER INGELHEIM GmbH (Merial)
- ELI-LILLY (Novartis Animal Health)
- Gene One Life Science
- GEOVAX LABS, INC
- Inovio Pharmaceuticals (VGX Animal Health)
- Genexine, Inc.
- VIATRIS INC. (Rottapharm Biotech)
- Takara Holdings (Takara Bio)
- ZOETIS INC. (Fort dodge Animal Health)
- Zydus Lifesciences Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | May 2025 |
| Forecast Period | 2024 - 2030 |
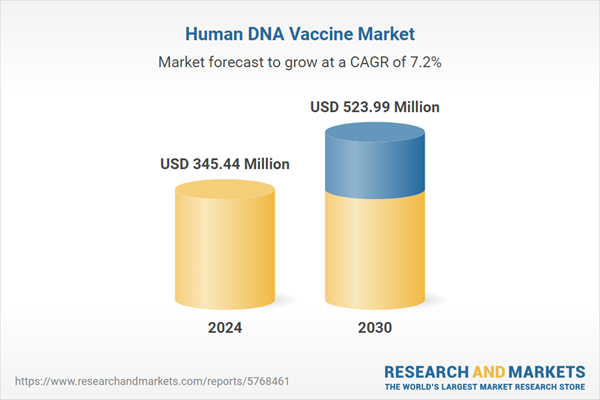
| Estimated Market Value ( USD | $ 345.44 Million |
| Forecasted Market Value ( USD | $ 523.99 Million |
| Compound Annual Growth Rate | 7.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |