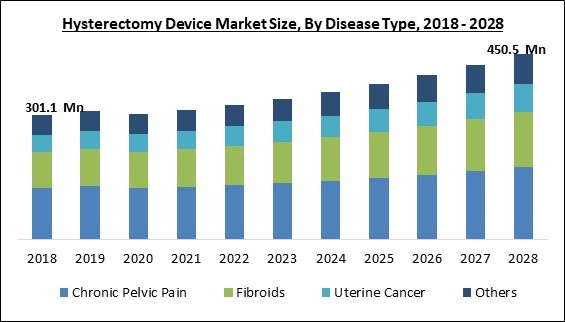
The Global Hysterectomy Device Market size is expected to reach $450.5 Million by 2028, rising at a market growth of 5.5% CAGR during the forecast period.
The hysterectomy is the name for the surgical surgery used to remove a woman's uterus. Moreover, this procedure can treat various chronic pain disorders, infections, and some forms of cancer. Depending on the goal of the procedure, a hysterectomy can take various forms. The entire uterus is typically removed in these operations. Hysterectomy can be used to treat uncontrollable vaginal bleeding, persistent pelvic pain, and cancer of the uterus, cervix, or ovaries.
Fibroids, which are benign tumors growing in the uterus, and uterine prolapse, which is when the uterus protrudes from the vagina through the cervix, can also be treated with it joined with adenomyosis, which is a condition in which the lining of the uterus grows into the muscles of the uterus, and pelvic inflammatory disease. A lower belly cut, commonly known as the abdomen, is used during an abdominal hysterectomy to remove the uterus, known as an open procedure.
While a woman is pregnant, the uterus, also known as the womb, is where the baby develops. The uterus is removed with a partial hysterectomy, but the womb's neck remains. The cervix is the womb's neck. Then, the uterus and cervix are removed during a complete hysterectomy. Moreover, a vaginal incision can be used to perform a hysterectomy which is called a vaginal hysterectomy. Alternatively, one might have robotic or laparoscopic surgery, which inserts long, thin equipment through tiny belly incisions.
In place of other hysterectomy procedures, one might require an abdominal hysterectomy if: the uterus is enormous; the doctor wants to examine other pelvic organs for illness symptoms; the doctor suggests an open procedure because it might be the best option for the patient, in their opinion. A hysterectomy may be the best course of treatment for uterine or cervical cancer. Other treatment options may include radiation or chemotherapy, depending on the type of cancer and its stage.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Medtronic PLC, B. Braun Melsungen AG, ConMed Corporation, Johnson & Johnson, Richard Wolf GmbH, Karl Storz SE & Co. KG, The Cooper Companies, Inc., Laborie Medical Technologies (Patricia Industries) (Investor AB), Fortimedix Surgical B.V., and Conkin Surgical Instruments Ltd.
The hysterectomy is the name for the surgical surgery used to remove a woman's uterus. Moreover, this procedure can treat various chronic pain disorders, infections, and some forms of cancer. Depending on the goal of the procedure, a hysterectomy can take various forms. The entire uterus is typically removed in these operations. Hysterectomy can be used to treat uncontrollable vaginal bleeding, persistent pelvic pain, and cancer of the uterus, cervix, or ovaries.
Fibroids, which are benign tumors growing in the uterus, and uterine prolapse, which is when the uterus protrudes from the vagina through the cervix, can also be treated with it joined with adenomyosis, which is a condition in which the lining of the uterus grows into the muscles of the uterus, and pelvic inflammatory disease. A lower belly cut, commonly known as the abdomen, is used during an abdominal hysterectomy to remove the uterus, known as an open procedure.
While a woman is pregnant, the uterus, also known as the womb, is where the baby develops. The uterus is removed with a partial hysterectomy, but the womb's neck remains. The cervix is the womb's neck. Then, the uterus and cervix are removed during a complete hysterectomy. Moreover, a vaginal incision can be used to perform a hysterectomy which is called a vaginal hysterectomy. Alternatively, one might have robotic or laparoscopic surgery, which inserts long, thin equipment through tiny belly incisions.
In place of other hysterectomy procedures, one might require an abdominal hysterectomy if: the uterus is enormous; the doctor wants to examine other pelvic organs for illness symptoms; the doctor suggests an open procedure because it might be the best option for the patient, in their opinion. A hysterectomy may be the best course of treatment for uterine or cervical cancer. Other treatment options may include radiation or chemotherapy, depending on the type of cancer and its stage.
COVID-19 Impact Analysis
The COVID-19 pandemic presented difficulties for healthcare practitioners around the world. As a result, resources were diverted from other healthcare services, including the industry for uterine manipulator devices, to care for COVID-19 patients. Many patients experienced significant delays in receiving gynecologic surgical care during the height of the pandemic. Many parts of numerous businesses, including product demand, operations, supply chains, distribution networks, and the capacity to conduct product research and development, are impacted by the COVID-19 pandemic. As a result, COVID-19 is anticipated to impact the market for hysterectomy device greatly in the initial period.Market Growth Factors
Rising prevalence of obesity
The prevalence of overweight and obesity among children and adolescents aged 5 to 19 has significantly grown, rising from roughly 4% in 1975 to a little over 18% in 2016. More than 124 million children and teens were obese in 2016, up from less than 1% in 1975 among those aged 5 to 19. Furthermore, women (40%) are more likely to be obese than men (35%), and women's health is majorly impacted by obesity in several distinct ways. Hence, the increasing obesity in women is expected to cause issues like fibroids, uterine cancer, or chronic pelvic pain that will increase the requirement for hysterectomies and boost the hysterectomy device market growth.The constant advancement of devices used in hysterectomy
Medical advancements have reduced the hazards of many surgeries to the point where they are now regarded as minimally invasive procedures. These developments have made hysterectomy surgery safer and less intrusive than ever for women who require this surgical removal of the uterus. A one-inch incision concealed in the belly button can now be used to perform hysterectomies due to the recent advancements in robotic surgery technology. The single-site robotic hysterectomy offers all the advantages of minimally invasive surgery and, in most cases, results in absolutely no scarring.Market Restraining Factors
Presence of medications and minimally invasive surgeries
The fibroids die and diminish within a few weeks to months once the blood supply is severed. This method might be better suitable for women who don't want to become pregnant shortly because studies have revealed a considerable risk of miscarriage and other pregnancy issues after this operation. Myomectomy, which allows for future pregnancies, is the surgical removal of the fibroids without removing the uterus. There is a chance of fibroid recurrence, like every other uterus-sparing operation. The availability of minimally invasive procedures, hormone therapies, and medicines might shift patients' preference toward them and restrict the hysterectomy device market growth.Disease Type Outlook
Based on disease type, the hysterectomy device market is segmented into uterine cancer, fibroids, chronic pelvic pain and others. The chronic pelvic pain segment held the highest revenue share in the hysterectomy device market in 2021. This is because it is a debilitating, persistent, chronic discomfort in a women’s pelvis. Chronic pelvic discomfort is frequently accompanied by comorbidities such as irritable bowel syndrome, major depressive illness, or pelvic inflammatory syndrome. Persistent pelvic discomfort is a complicated illness with several potential causes.End-user Outlook
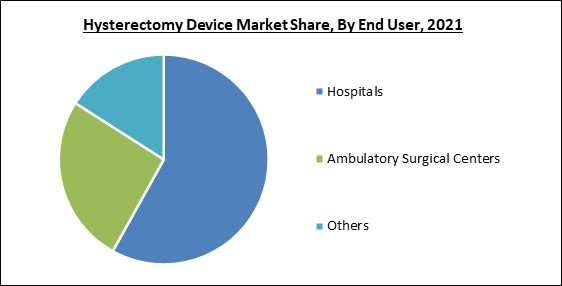
On the basis of end user, the hysterectomy device market is divided into hospitals, ambulatory surgical centers and others. The ambulatory surgical centers segment procured a substantial revenue share in the hysterectomy device market in 2021. The growth is attributed to several factors, including a high desire for outpatient treatments, an increase in the use of minimally invasive procedures, and the affordability of laparoscopic procedures performed in ambulatory surgery centers. The demand for mobile surgery facilities is anticipated to rise due to the lower risk of postoperative complications in minimally invasive laparoscopic surgery.Surgical Approach Outlook
By surgical approach, the hysterectomy device market is classified into robotic assisted laparoscopic hysterectomy, abdominal hysterectomy, vaginal hysterectomy and laparoscopic hysterectomy. The abdominal hysterectomy segment registered the highest revenue share in the hysterectomy device market in 2021. This is owing to the benefits like improving the quality of life, particularly if the patient suffers from persistent pelvic discomfort or heavy, irregular bleeding. Through a six- to eight-inch abdominal incision, the uterus is removed. The incision is either done from the navel to the pubic bone or along the top of the pubic hairline.Regional Outlook
Region-wise, the hysterectomy device market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America region led the hysterectomy device market by generating the maximum revenue share in 2021. This is due to several significant players and technical improvements in the region. These leading players play an essential role in acquisition strategies. Furthermore, the region's rising uterine cancer prevalence, strong consumer base, spending power, and established healthcare infrastructure are anticipated to fuel the hysterectomy device market expansion in the region.The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Medtronic PLC, B. Braun Melsungen AG, ConMed Corporation, Johnson & Johnson, Richard Wolf GmbH, Karl Storz SE & Co. KG, The Cooper Companies, Inc., Laborie Medical Technologies (Patricia Industries) (Investor AB), Fortimedix Surgical B.V., and Conkin Surgical Instruments Ltd.
Strategies Deployed in Hysterectomy Device Market
- Feb-2023: Laborie announced the acquisition of Novonate and its LifeBubble technology, a device used to raise the foetal head, making birth easier and less traumatic for the mother and baby. Together, the companies are focused on advancing the standard of care for neonates in intensive care.
- Nov-2022: Medtronic Private Limited, a division of Medtronic PLC announced the launch of the TruClear system, a mechanical hysteroscopic tissue removal for efficient and safe treatment of intra-uterine anomalies (IUA). Unlike other intrauterine abnormality therapy methods, the TruClear device eliminates intrauterine tissue manually instead of employing a high-frequency electric current. The device is intended to facilitate the treatment of intrauterine illnesses in a less invasive and efficient manner, giving patients the choice to maintain the organ and swiftly return to their regular lives.
- Sep-2022: Medtronic together with CARE Hospitals Group, introduced the first gynecology (hysterectomy) procedure in the Asia Pacific leveraging the Hugo robotic-assisted surgery (RAS) system. To provide high-quality patient care at reasonable prices, investments in technology-enabled healthcare solutions are essential. High-quality equipment, like a robotic system, aids in accuracy improvement, shortens hospital stays, and speeds up patient recuperation.
- Feb-2022: CooperCompanies signed an agreement to acquire Cook Medical's Reproductive Health Business, a company engaged in manufacturing minimally invasive medical devices for obstetrics, fertility, and gynecology markets. The acquisition added delivery devices to the former company's ObGyn portfolio and would expand its international fertility footprint.
- Feb-2022: Laborie acquired Clinical Innovations for continuing to support moms, babies, and medical professionals all over the world by investing in and expanding Clinical Innovations' distinctive product line and specialist channels. The company's ability to serve the specific customer call point of obstetricians, gynecologists, neonatologists, and NICU and L&D nurses will be enhanced by Clinical Innovations' already strong and trusted relationships with physicians and nurses.
- Dec-2021: CooperCompanies acquired Generate Life Sciences, a provider of donor sperm and egg for fertility cryopreservation service, fertility treatments, and newborn stem cell storage. With this acquisition, the company would be able to offer fertility clinics and OB/GYNs an even stronger service.
- May-2021: CooperSurgical took over obp Medical Corporation, a company that focuses on developing and marketing diversified products comprising single-use vaginal speculums with combined LED illumination. The acquisition complemented CooperSurgical's portfolio of OB/GYN medical devices.
- Mar-2021: CooperSurgical completed the acquisition of Safe Obstetric Systems, a company engaged in manufacturing medical devices, Fetal Pillow, an FDA-approved product used to raise the foetal head, making the birth easier and less traumatic for the mother and baby, after a fully dilated caesarean section. The acquisition is a perfect fit for CooperSurgical's mission of women's healthcare advancement.
Scope of the Study
By Disease Type
- Chronic Pelvic Pain
- Fibroids
- Uterine Cancer
- Others
By End-user
- Hospitals
- Ambulatory Surgical Centers
- Others
By Surgical Approach
- Abdominal Hysterectomy
- Laparoscopic Hysterectomy
- Robotic-assisted Laparoscopic Hysterectomy
- Vaginal Hysterectomy
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Medtronic PLC
- B.Braun Melsungen AG
- ConMed Corporation
- Johnson & Johnson
- Richard Wolf GmbH
- Karl Storz SE & Co. KG
- The Cooper Companies, Inc.
- Laborie Medical Technologies ( Patricia Industries)(Investor AB)
- Fortimedix Surgical B.V.
- Conkin Surgical Instruments Ltd
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Chapter 1. Market Scope & Methodology
Chapter 2. Market Overview
Chapter 4. Global Hysterectomy Device Market by Disease Type
Chapter 5. Global Hysterectomy Device Market by End-user
Chapter 6. Global Hysterectomy Device Market by Surgical Approach
Chapter 7. Global Hysterectomy Device Market by Region
Chapter 8. Company Profiles
Companies Mentioned
- Medtronic PLC
- B. Braun Melsungen AG
- ConMed Corporation
- Johnson & Johnson
- Richard Wolf GmbH
- Karl Storz SE & Co. KG
- The Cooper Companies, Inc.
- Laborie Medical Technologies ( Patricia Industries)(Investor AB)
- Fortimedix Surgical B.V.
- Conkin Surgical Instruments Ltd