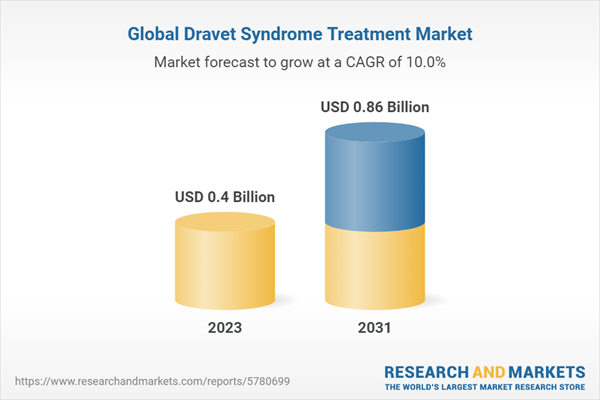
The growth of the global Dravet syndrome treatment size is being driven by the rising prevalence of the condition across the globe. The global Dravet syndrome treatment market is likely to grow at a rate of 10.04% in the forecast period of 2023-2031 to attain a value of USD 0.86 billion by 2031.
The prevalence of Dravet Syndrome varies by region, but it is estimated to affect approximately one in every 20,000 to 40,000 individuals worldwide. It is more common in males than females, with a male-to-female ratio of approximately 1.5:1.
The treatment options for Dravet Syndrome are focused on managing the symptoms of the disease and improving the quality of life of patients. The primary treatment is medication, specifically anticonvulsants such as clobazam, valproate, and stiripentol. These drugs work by stabilizing the electrical activity in the brain, reducing the frequency and severity of seizures. Benzodiazepines may also be used in combination with anticonvulsants to manage acute seizures.
North America dominates the Dravet Syndrome treatment market, followed by Europe and Asia Pacific. The high prevalence of Dravet Syndrome in North America, coupled with the favorable reimbursement policies, is driving the growth of the market in the region. Europe is also a significant market for Dravet Syndrome treatment, with the presence of key players and increasing investment in research and development activities. The Asia Pacific region is expected to grow at the highest CAGR during the forecast period, owing to the increasing awareness about the disease and the rising healthcare expenditure in the region.
Dravet Syndrome Treatment Market: Introduction
Dravet Syndrome, also known as Severe Myoclonic Epilepsy of Infancy (SMEI), is a rare, severe form of epilepsy that starts in infancy. It is a genetic disorder caused by mutations in the SCN1A gene. There is currently no cure for Dravet Syndrome, but there are various treatment options available that aim to manage the symptoms of the disease. The report covers the market for Dravet Syndrome treatment, including drugs and therapies, and provides a detailed analysis of the market size, growth, and trends.Dravet Syndrome Epidemiology
Dravet Syndrome, also known as Severe Myoclonic Epilepsy of Infancy (SMEI), is a rare genetic disorder that affects approximately one in every 15,700 individuals worldwide. The disease typically begins in infancy, with infants experiencing seizures that are often prolonged and difficult to manage. As the child grows, the seizures may become more frequent and severe, and may be accompanied by cognitive and behavioral problems.The prevalence of Dravet Syndrome varies by region, but it is estimated to affect approximately one in every 20,000 to 40,000 individuals worldwide. It is more common in males than females, with a male-to-female ratio of approximately 1.5:1.
The treatment options for Dravet Syndrome are focused on managing the symptoms of the disease and improving the quality of life of patients. The primary treatment is medication, specifically anticonvulsants such as clobazam, valproate, and stiripentol. These drugs work by stabilizing the electrical activity in the brain, reducing the frequency and severity of seizures. Benzodiazepines may also be used in combination with anticonvulsants to manage acute seizures.
DRAVET SYNDROME TREATMENT Market Segmentations
The market can be categorised into seizure type, diagnosis type, treatment type, end user, and major region.Market Breakup by Seizures Type
- Myoclonic Seizures
- Atonic Seizures
- Partial Seizures
- Absence Seizures
- Tonic Seizures
- Photosensitive Seizures
- Others
Market Breakup by Diagnosis Type
- Magnetic Resonance Imaging
- Electroencephalography
- SCN1 A Testing
- Others
Market Breakup by Treatment Type
- Seizure Medications
- Ketogenic Diet
- Vagus Nerve Stimulation
- Others
Market Breakup by End User
- Pharmaceutical Companies
- Hospitals
- Diagnostic Laboratories
- Academic and Research Institutes
- Others
Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Dravet Syndrome Treatment Market Scenario
The global Dravet syndrome treatment market is expected to grow at a significant rate during the forecast period of 2023-2031. The increasing prevalence of the disease, rising awareness, and growing investment in research and development activities are the key drivers of the market. North America dominates the market, followed by Europe and Asia Pacific. The leading players in the market are focusing on the development of new drugs and therapies and adopting various strategies to strengthen their position in the market.North America dominates the Dravet Syndrome treatment market, followed by Europe and Asia Pacific. The high prevalence of Dravet Syndrome in North America, coupled with the favorable reimbursement policies, is driving the growth of the market in the region. Europe is also a significant market for Dravet Syndrome treatment, with the presence of key players and increasing investment in research and development activities. The Asia Pacific region is expected to grow at the highest CAGR during the forecast period, owing to the increasing awareness about the disease and the rising healthcare expenditure in the region.
Key Players in the Global Dravet Syndrome Treatment Market
The report gives an in-depth analysis of the key players involved in the Dravet Syndrome Treatment market, sponsors manufacturing the drugs, and putting them through trials to get FDA approvals. The companies included in the market are as follows:- Biocodex
- Biscayne Neurotherapeutics (Supernus Pharmaceuticals, Inc)
- Cyberonics (LivaNova PLC)
- Epygenix Therapeutics, Inc
- GW Pharmaceuticals (Jazz Pharmaceuticals, Inc)
- OPKO Health, Inc
- Ovid Therapeutics
- PTC Therapeutics
- Encoded Therapeutics, Inc
- Zogenix (UCB S.A, Belgium)
- Takeda Pharmaceutical Company Limited
Table of Contents
1 Preface
4 Global Dravet Syndrome Overview
5 Patient Profile
6 Current Scenario Evaluation and Regulatory Framework
7 Challenges and Unmet Needs
8 Global Dravet Syndrome Treatment Market
9 North America Dravet Syndrome Treatment Market
10 Europe Dravet Syndrome Treatment Market
11 Asia Pacific Dravet Syndrome Treatment Market
12 Latin America Dravet Syndrome Treatment Market
13 Middle East and Africa Dravet Syndrome Treatment Market
14 Global Dravet Syndrome Treatment Market Dynamics
15 Supplier Landscape
16 Global Dravet Syndrome Treatment Market Distribution Model (Additional Insight)
17 Payment Methods (Additional Insight)
Companies Mentioned
- Biocodex
- Biscayne Neurotherapeutics (Supernus Pharmaceuticals, Inc.)
- Cyberonics (LivaNova PLC)
- Epygenix Therapeutics, Inc.
- GW Pharmaceuticals (Jazz Pharmaceuticals, Inc.)
- OPKO Health, Inc.
- Ovid Therapeutics
- PTC Therapeutics
- Encoded Therapeutics, Inc.
- Zogenix (UCB S.A., Belgium)
- Takeda Pharmaceutical Company Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 147 |
| Published | April 2023 |
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 0.4 Billion |
| Forecasted Market Value ( USD | $ 0.86 Billion |
| Compound Annual Growth Rate | 10.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |