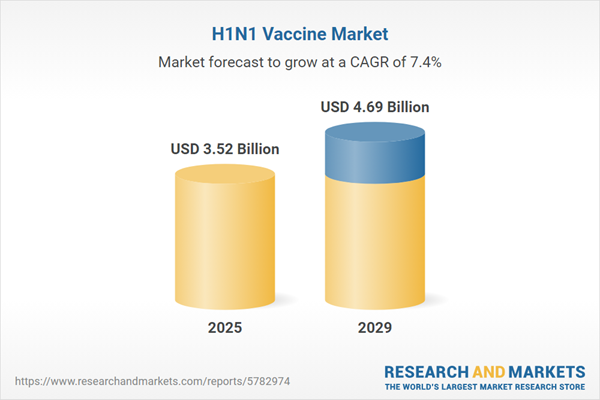
The H1N1 vaccine market size is expected to see strong growth in the next few years. It will grow to $4.69 billion in 2029 at a compound annual growth rate (CAGR) of 7.4%. The growth in the forecast period can be attributed to research on broad-spectrum vaccines, global vaccine equity initiatives, public health resilience building, global health governance strengthening, integration of h1n1 vaccination with routine immunization. Major trends in the forecast period include global pandemic preparedness, influenza vaccination programs, vaccine development advances, cross-protection research, global health initiatives, technological improvements in vaccine manufacturing, production capacity expansion, data monitoring and surveillance.
The forecast of 7.4% growth over the next five years reflects a modest reduction of 0.3% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Trade tensions could disrupt U.S. pandemic preparedness by inflating costs of influenza antigen production and single-dose prefilled syringes imported from the Netherlands and South Korea, delaying mass vaccination campaigns and raising public health emergency response costs. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The rising investment in research and development activities related to immunology and vaccines is expected to drive the growth of the H1N1 vaccine market in the future. Immunology and vaccines play essential roles in preventing infections and promoting the production of antibodies to combat them. This field is vital in medical and biological research, as it seeks to understand the immune system better. The H1N1 vaccine is specifically designed to enhance antibody production, providing immunity against various diseases. For instance, in March 2023, the National Institutes of Health (NIH), a U.S.-based agency, reported that its research had facilitated the rapid development of mRNA COVID-19 vaccines. By March 2022, over 577 million doses had been administered in the United States, saving an estimated 2.4 million lives and preventing 17 million hospitalizations. Therefore, the growing expenditure on research and development in immunology and vaccines is significantly contributing to the expansion of the H1N1 vaccine market.
The surge in international travel is a catalyst for the H1N1 vaccine market. International travel, involving cross-border movement for tourism, business, and more, demands H1N1 vaccination as a preventive measure. Nations and global bodies recommend or mandate H1N1 vaccination to curb virus transmission during travel. International tourism receipts hitting $1 trillion in 2022 and reaching 64% of pre-pandemic levels signify the market's growth fueled by increased travel safety measures.
Strategic collaborations among companies are emerging as a significant trend in the H1N1 (swine flu) vaccination market. Firms in this sector are increasingly focusing on partnerships to enhance their market share. For instance, in August 2022, a prominent pharmaceutical company and hVIVO, an affiliate of Open Orphan, entered into a $123 million agreement to develop a new batch of H1N1 influenza challenge virus. This collaboration has bolstered the growth potential for both companies within the industry.
Innovations like needle-free nasal vaccines propel the H1N1 vaccine market. Needle-free nasal vaccines, devoid of traditional needles or syringes, offer a painless, non-invasive immunization method, predominantly through nasal administration. Nasovac S4, launched by Serum Institute and Mylab in October 2023, targets H1N1 and H3N2 viruses, stimulating immune response in individuals aged 2 and above against influenza strains A and B, underscoring market sustainability and product advancements.
In July 2023, Sinovac Biotech Ltd., a prominent biopharmaceutical company based in China, forged a strategic partnership with Bio Farma, a state-owned biopharmaceutical company based in Indonesia, for an undisclosed investment. This alliance is designed to cater to Indonesia's domestic vaccine market while targeting expansion into the international sphere. The collaboration between Sinovac and Bio Farma is aimed at facilitating Sinovac's introduction of upcoming products, such as varicella, influenza, and pneumococcal polysaccharide vaccines. These initiatives are intended to address the healthcare needs of the region effectively and contribute to the enhancement of local and national public health initiatives. Bio Farma is an Indonesia-based state-owned biopharmaceutical company, operating in various vaccine markets, including H1N1.
Major companies operating in the H1N1 vaccine market are Sanofi SA, GlaxoSmithKline PLC, CSL Limited, AstraZeneca Inc., Zydus Lifesciences Limited, Merck and Co.Inc., Novavax Inc., Panacea Biotec Ltd., Sinovac Biotech Ltd., Green Cross Corp., Pfizer Inc., Seqirus Ltd., Cipla Inc., Protein Sciences Corporation, Hualan Biological Engineering Inc., Changsheng Bio-Technology Co. Ltd., Bharat Biotech International Limited, Serum Institute of India Pvt. Ltd., Bio Farma Group, PT Kalbe Farma Tbk, Moderna Inc., CureVac NV, BioNTech SE, Inovio Pharmaceuticals Inc., Vaxart Inc., Altimmune Inc., Dynavax Technologies Corporation, VBI Vaccines Inc., GeoVax Labs Inc.
North America was the largest region in the H1N1 vaccine market in 2024. The regions covered in the h1n1 vaccine market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the h1n1 vaccine market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The H1N1 vaccine market report is one of a series of new reports that provides H1N1 vaccine market statistics, including H1N1 vaccine industry global market size, regional shares, competitors with an H1N1 vaccine market share, detailed H1N1 vaccine market segments, market trends and opportunities, and any further data you may need to thrive in the H1N1 vaccine industry. This H1N1 vaccine market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
H1N1 vaccines are designed for individuals aged six months and older to guard against influenza triggered by the H1N1 2009 virus. These vaccines work by introducing a small, weakened dose of the virus into the body, stimulating the immune system to build protection against the virus.
The primary product types for H1N1 vaccines include inactivated and live attenuated vaccines. Inactivated vaccines within the market are formulated to prevent diseases caused by the influenza H1N1 virus. They consist of synthetic vaccines with protective immunizing properties, containing hemagglutinin (HA) antigens derived from four inactivated influenza viruses, encompassing two distinct influenza type A strains and two different influenza type B strains. These vaccines are administered through various routes such as intradermal, intramuscular, and subcutaneous methods, and they are utilized across hospitals, clinics, research and diagnostic laboratories, among other medical settings.
The H1N1 vaccines market consists of sales of TIV (flu shot (injection) and LAIV (nasal spray (mist) of live attenuated influenza vaccine). Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
H1N1 Vaccine Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on h1n1 vaccine market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for h1n1 vaccine? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The h1n1 vaccine market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product Type: Inactivated Vaccine; Live Attenuated Vaccine2) By Route of Administration: Intradermal Vaccination; Intramuscular Vaccination; Subcutaneous Vaccination
3) By End Users: Hospitals; Clinics; Research and Diagnostic Laboratories; Other End Users
Subsegments:
1) By Inactivated Vaccine: Injectable Inactivated Vaccine; Intranasal Inactivated Vaccine2) By Live Attenuated Vaccine: Injectable Live Attenuated Vaccine; Intranasal Live Attenuated Vaccine
Companies Mentioned: Sanofi SA; GlaxoSmithKline PLC; CSL Limited; AstraZeneca Inc.; Zydus Lifesciences Limited; Merck and Co.Inc.; Novavax Inc.; Panacea Biotec Ltd.; Sinovac Biotech Ltd.; Green Cross Corp.; Pfizer Inc.; Seqirus Ltd.; Cipla Inc.; Protein Sciences Corporation; Hualan Biological Engineering Inc.; Changsheng Bio-Technology Co. Ltd.; Bharat Biotech International Limited; Serum Institute of India Pvt. Ltd.; Bio Farma Group; PT Kalbe Farma Tbk; Moderna Inc.; CureVac NV; BioNTech SE; Inovio Pharmaceuticals Inc.; Vaxart Inc.; Altimmune Inc.; Dynavax Technologies Corporation; VBI Vaccines Inc.; GeoVax Labs Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this H1N1 Vaccine market report include:- Sanofi SA
- GlaxoSmithKline PLC
- CSL Limited
- AstraZeneca Inc.
- Zydus Lifesciences Limited
- Merck and Co.Inc.
- Novavax Inc.
- Panacea Biotec Ltd.
- Sinovac Biotech Ltd.
- Green Cross Corp.
- Pfizer Inc.
- Seqirus Ltd.
- Cipla Inc.
- Protein Sciences Corporation
- Hualan Biological Engineering Inc.
- Changsheng Bio-Technology Co. Ltd.
- Bharat Biotech International Limited
- Serum Institute of India Pvt. Ltd.
- Bio Farma Group
- PT Kalbe Farma Tbk
- Moderna Inc.
- CureVac NV
- BioNTech SE
- Inovio Pharmaceuticals Inc.
- Vaxart Inc.
- Altimmune Inc.
- Dynavax Technologies Corporation
- VBI Vaccines Inc.
- GeoVax Labs Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.52 Billion |
| Forecasted Market Value ( USD | $ 4.69 Billion |
| Compound Annual Growth Rate | 7.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |