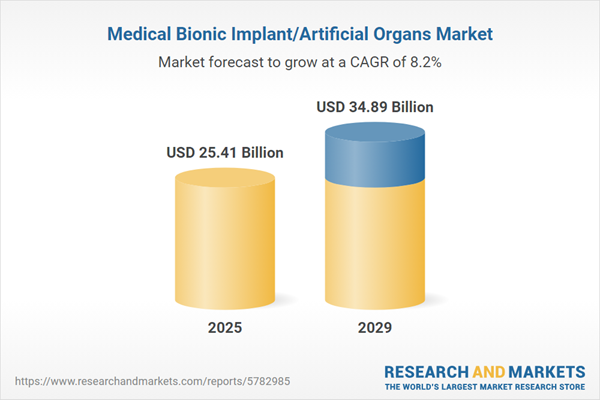
The medical bionic implant/artificial organs market size is expected to see strong growth in the next few years. It will grow to $34.89 billion in 2029 at a compound annual growth rate (CAGR) of 8.2%. The growth in the forecast period can be attributed to bioprinting and tissue engineering, emergence of biohybrid implants, focus on cardiac bionics, enhanced sensory feedback, regenerative medicine in bionics. Major trends in the forecast period include advancements in prosthetics and orthotics, increasing focus on brain-machine interfaces (BMIS), bionic organs for transplantation, 3D printing in bionic implants, integration of artificial intelligence (AI), wireless connectivity and remote monitoring.
The increase in road accidents and injuries is anticipated to drive the growth of the medical bionic implant and artificial organs market in the coming years. Road accidents refer to incidents on public roads that result in injuries to victims. Artificial organ and tissue replacements are performed to address organ damage primarily caused by traumatic events such as vehicle accidents, violent crimes, work-related injuries, or difficult births. For example, in May 2022, the National Highway Traffic Safety Administration (NHTSA), a US federal government agency focused on transportation safety, reported a 10.5% rise in motor vehicle crash fatalities, estimating approximately 42,915 deaths compared to 38,824 over the previous two years. Consequently, the increase in road accidents and injuries is driving the expansion of the medical bionic implant and artificial organs market.
The increasing prevalence of chronic diseases is poised to propel growth within the medical bionic implant and artificial organs sector. Chronic diseases persisting for three months or more are becoming more widespread, necessitating the use of medical bionic implants and artificial organs to manage conditions like diabetes. For example, projections from the National Library of Medicine indicate a 99.5% increase in individuals aged 50 and above with at least one chronic condition in the US by 2050. Moreover, as highlighted in September 2022 by the World Health Organization, approximately 41 million deaths each year globally are attributed to noncommunicable diseases or chronic ailments, emphasizing the market growth driven by the mounting prevalence of chronic diseases.
Strategic partnerships are becoming a prominent trend in the medical bionic implant and artificial organs market. Many companies in this sector are forming partnerships to strengthen their market positions. For example, in September 2024, Beta Bionics, a US-based medical equipment manufacturing company, collaborated with Abbott Laboratories, a US-based medical device firm, to integrate the iLet Bionic Pancreas with the FreeStyle Libre 3 continuous glucose monitoring (CGM) system. This collaboration aims to enhance diabetes management for users of the iLet Bionic Pancreas by providing seamless integration with Abbott's advanced CGM technology. Additionally, in February 2022, HUMOTECH, a US-based research services company focused on artificial organs, partnered with the University of Michigan, a public research university in the US. This partnership seeks to accelerate the development of bionic leg control software, which could provide prosthetic users with the strength and natural gait of a human leg. As part of this collaboration, HUMOTECH is developing updated versions of the open-source leg to better serve prosthesis users and researchers.
Major players in the medical bionic implant and artificial organs market are leveraging CAD and 3D printing technologies to gain a competitive edge. Utilizing Computer-Aided Design (CAD) and 3D printing technologies allows for precise customization and rapid prototyping of medical bionic implants, particularly in crafting artificial organs. For instance, CureTech's launch of the Polyether ether ketone (PEEK) cranial implant in April 2022 demonstrates the application of CAD software and 3D printing to create a biocompatible implant used in orthopedic applications like spinal fusion, joint replacement, and trauma surgery.
In May 2022, LivaNova PLC, a UK-centered medical device manufacturer, completed the acquisition of ALung Technologies, Inc., for an undisclosed sum. Foreseen to maintain a neutral impact on adjusted diluted earnings per share in 2022, this move signifies LivaNova's commitment to strategic portfolio investments. It includes the phased release of the next-generation heart-lung machine, Essenz, and the broadened applications in advanced circulatory procedures. ALung Technologies Inc., situated in the United States, specializes in the production of artificial organs.
Major companies operating in the medical bionic implant/artificial organs market are Medtronic PLC, Boston Scientific Services Private Limited., Abbott Laboratories Inc., Zimmer Biomet Holdings Inc., Ekso Bionics Holdings Inc., Cochlear Limited, Cyberonics Inc., LivaNova PLC, NeuroPace Inc., Ossur India Private Limited, Vivani Medical Inc., St Jude Medical India Private Limited, LifeNet Health Inc., Ottobock SE & Co. KGaA, Edwards Lifesciences India Pvt. Ltd., SynCardia Systems LLC, Demant A/S, Berlin Heart GmbH, Conmed Devices Private Limited, Wright Medical Group N.V., Abiomed Inc., Baxter Laboratories Inc., Asahi Kasei Medical Co. Ltd., Getinge AB, Bornlife Prosthetic and Orthotic Inc., Sonova Holding AG, Biocontrol Medical Ltd., Orthofix International Inc., Aleva Neurotherapeutics SA, Second Sight Medical Products Inc., Touch Bionics Inc., Thoratec Corporation, CardiacAssist Inc., HeartWare International Inc., Synapse Biomedical Inc., Neurotech NA Inc., Retina Implant AG, Advanced Bionics AG, Med-El Elektromedizinische Geräte GmbH.
Asia-Pacific was the largest region in the medical bionic implant/artificial organs market share in 2024. The regions covered in the medical bionic implant/artificial organs market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the medical bionic implant/artificial organs market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Medical bionic implant artificial organs encompass devices or machinery utilized to replicate the functions of a damaged or missing body organ or part. These innovations serve to restore physical capabilities to individuals facing disabilities, spanning a range of implants that notably enhance cardiac and neurological functions.
The realm of medical bionic implants and artificial organs, two primary categories exist such as bionic implants and artificial organs. Bionic implants consist of electronic or mechatronic elements designed to augment or reinstate the physical abilities of individuals with disabilities. These implants vary in fixation methods, including implantable and externally worn options, leveraging electronic and mechanical technologies. They find applications across diverse settings such as hospitals, clinics, research institutes, academic institutions, and beyond.
The medical bionic implant/artificial organs market research report is one of a series of new reports that provides medical bionic implant/artificial organs market statistics, including medical bionic implant/artificial organs industry global market size, regional shares, competitors with a medical bionic implant/artificial organs market share, detailed medical bionic implant/artificial organs market segments, market trends and opportunities, and any further data you may need to thrive in the medical bionic implant/artificial organs industry. This medical bionic implant/artificial organs market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The medical bionic implant artificial organs market consists of sales of pacemakers, ventricular assist device, artificial limb and fingers, exoskeletons, heart valves. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Medical Bionic Implant/Artificial Organs Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on medical bionic implant/artificial organs market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for medical bionic implant/artificial organs? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The medical bionic implant/artificial organs market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) by Type: Bionic Implants; Artificial Organs2) by Method of Fixation: Implantable; Externally Worn
3) by Technology: Electronic; Mechanical
4) by Application: Hospitals; Clinics; Research and Academic Institutes; Other Applications
Subsegments:
1) by Bionic Implants: Bionic Limbs; Bionic Eyes; Cochlear Implants; Artificial Organs2) by Artificial Organs: Artificial Hearts; Artificial Kidneys; Artificial Livers; Artificial Pancreas
Key Companies Mentioned: Medtronic PLC; Boston Scientific Services Private Limited.; Abbott Laboratories Inc.; Zimmer Biomet Holdings Inc.; Ekso Bionics Holdings Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Medical Bionic Implant/Artificial Organs market report include:- Medtronic PLC
- Boston Scientific Services Private Limited.
- Abbott Laboratories Inc.
- Zimmer Biomet Holdings Inc.
- Ekso Bionics Holdings Inc.
- Cochlear Limited
- Cyberonics Inc.
- LivaNova PLC
- NeuroPace Inc.
- Ossur India Private Limited
- Vivani Medical Inc.
- St Jude Medical India Private Limited
- LifeNet Health Inc.
- Ottobock SE & Co. KGaA
- Edwards Lifesciences India Pvt. Ltd.
- SynCardia Systems LLC
- Demant A/S
- Berlin Heart GmbH
- Conmed Devices Private Limited
- Wright Medical Group N.V.
- Abiomed Inc.
- Baxter Laboratories Inc.
- Asahi Kasei Medical Co. Ltd.
- Getinge AB
- Bornlife Prosthetic and Orthotic Inc.
- Sonova Holding AG
- Biocontrol Medical Ltd.
- Orthofix International Inc.
- Aleva Neurotherapeutics SA
- Second Sight Medical Products Inc.
- Touch Bionics Inc.
- Thoratec Corporation
- CardiacAssist Inc.
- HeartWare International Inc.
- Synapse Biomedical Inc.
- Neurotech NA Inc.
- Retina Implant AG
- Advanced Bionics AG
- Med-El Elektromedizinische Geräte GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 25.41 Billion |
| Forecasted Market Value ( USD | $ 34.89 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 39 |