Cardiac Rhythm Management Devices Market: Introduction
Cardiac rhythm management (CRM) devices are medical devices used to diagnose and treat abnormal heart rhythms, or arrhythmias. These devices include pacemakers, implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization therapy (CRT) devices. CRM devices are designed to monitor the heart's electrical activity, deliver electrical impulses to regulate the heart rate, and provide therapy to prevent sudden cardiac arrest.Pacemakers are used to treat bradycardia, a condition where the heart beats too slowly. The device monitors the heart's electrical activity and delivers electrical impulses to the heart muscle to help it beat regularly and at a consistent rate.
ICDs are used to treat life-threatening arrhythmias, such as ventricular fibrillation and ventricular tachycardia, which can lead to sudden cardiac arrest. The device monitors the heart's electrical activity and delivers a shock to the heart to restore a normal rhythm if an abnormal rhythm is detected.
CRT devices are used to treat heart failure and are designed to improve the heart's pumping efficiency. The device synchronizes the contractions of the heart's ventricles by sending electrical impulses to the heart muscle.
Overall, CRM devices play a crucial role in the diagnosis and treatment of heart rhythm disorders, improving the quality of life for patients and reducing the risk of life-threatening complications. These devices have advanced significantly over the years, with improved technology and features, such as remote monitoring and wireless connectivity, making them more effective and convenient for patients.
Cardiac Rhythm Management Devices Market Segmentations
Market Breakup by Product Type
- Pacemakers
- Implantable
- External
- Defibrillators
- Implantable Cardioverter Defibrillators (ICD)
- S-ICD
- T-ICD
- External Defibrillator
- Manual External Defibrillator
- Automatic External Defibrillator
- Wearable Cardioverter Defibrillator
- Cardiac Resynchronization Therapy (CRT)
- CRT-Defibrillator
- CRT-Pacemakers
- Others
Market Breakup by Applications
- Bradycardia
- Tachycardia
- Heart Failure
- Others
Market Breakup by End User
- Hospital
- Home Care Settings
- Ambulatory Care Settings
- Others
Cardiac Rhythm Management Devices Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Cardiac Rhythm Management Devices Market Scenario
The cardiac rhythm management (CRM) devices market is a growing segment of the medical device industry. The increasing prevalence of heart rhythm disorders, such as atrial fibrillation and heart failure, has contributed to the growth of the market. Additionally, the rise in the geriatric population and the adoption of unhealthy lifestyles have further increased the demand for CRM devices.Advancements in CRM device technology have led to the development of smaller, more reliable, and more advanced devices that offer longer battery life and more sophisticated programming options. The integration of wireless connectivity and remote monitoring capabilities in CRM devices has also improved patient outcomes by enabling physicians to monitor patients' heart rhythm remotely and adjust the device settings accordingly.
The market is witnessing partnerships and collaborations among companies to expand their product offerings and reach a wider customer base. The CRM devices market is highly competitive, with several players offering a range of products and services. The market is also seeing an increasing trend of customization, with CRM devices being designed to meet the unique needs of individual patients.
Moreover, the COVID-19 pandemic has accelerated the adoption of telehealth services, including remote cardiac monitoring and CRM device monitoring. This has led to an increase in the use of remote monitoring devices and telehealth services, which enable remote monitoring and consultation with healthcare professionals.
Overall, the CRM devices market is expected to continue growing in the coming years, driven by technological advancements, rising demand for remote monitoring, and increasing partnerships among players in the market. The market is poised for growth as healthcare providers and patients continue to recognize the benefits of CRM therapy for managing heart rhythm disorders.
Key Players in the Global Cardiac Rhythm Management Devices Market
The report gives an in-depth analysis of the key players involved in the cardiac rhythm management devices market, sponsors manufacturing the drugs, and putting them through trials to get FDA approvals. The companies included in the market are as follows:- Physio-Control, Inc
- Schiller Americas, Inc
- Medtronic
- Abbott
- Boston Scientific Corporation
- BIOTRONIK SE & Co. KG
- GE Healthcare
- Asahi Kasei Corporation
- MicroPort Scientific Corporation
- NIHON KOHDEN CORPORATION
- BPL Medical Technologies
- Lepu Medical Technology
- Pacetronix
- Osypka Medical
- AliveCor, Inc.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Physio-Control, Inc.
- Schiller Americas, Inc.
- Medtronic
- Abbott
- Boston Scientific Corporation
- Biotronik Se & Co. Kg
- Ge Healthcare
- Asahi Kasei Corporation
- Microport Scientific Corporation
- Nihon Kohden Corporation
- Bpl Medical Technologies
- Lepu Medical Technology
- Pacetronix
- Osypka Medical
- Alivecor, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | April 2023 |
| Forecast Period | 2023 - 2031 |
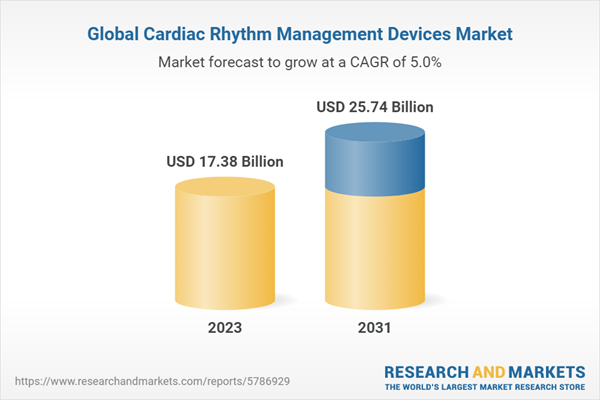
| Estimated Market Value ( USD | $ 17.38 Billion |
| Forecasted Market Value ( USD | $ 25.74 Billion |
| Compound Annual Growth Rate | 5.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |