Global C-arms Market Overview
C-arms is a medical imaging device with fluoroscopy and radiography capabilities. The equipment has a C-shaped arm, connected to an X-ray source at one end and a detector at the other. C-arms provide X-ray images in real-time, enabling surgeons to monitor anatomical details while performing complicated surgical procedures including orthopedic, gastroenterology, and urology surgeries. Mobile C-arms provide great flexibility to capture high-resolution X-ray images of a patient's body from different angles, improving accuracy and facilitating better patient outcomes.Robust imaging systems like C-arms are essential for the success of complex surgeries such as knee replacement and hip replacement orthopedic procedures. It is estimated that around 790,000 total knee replacement and more than 450,000 hip replacement surgeries are performed by orthopedic surgeons annually in the United States. The number of such surgeries is expected to surge in the coming years which will bolster the market growth of C-arms devices in the forecast period.
Advancements in the C-arm systems are the driving force behind the development of minimally invasive surgeries. With the rising demand for minimally invasive procedures rising, the C-arms market share is poised to witness considerable growth. In addition, the increasing incidence of geriatric population and prevalence of chronic diseases are fuelling the need for technologically advanced C-arms devices.
Rise in High-Quality Product Launches to Meet C-arms Market Demand
To develop versatile C-arms with optimal image acquisition and patient outcomes, the key market players are launching high-quality imaging systems to accommodate the growing market demand. In May 2023, Royal Philips, a leading health technology headquartered in the Netherlands, launched the Zenition 10 mobile C-arm system, expanding the portfolio of its Zenition mobile C-arm series. The surgical imaging system features enhanced imaging capabilities such as high-quality flat panel imaging and ease of use in routine surgical procedures. The cost-effective mobile imaging device is predicted to increase access to high-quality patient care at a lower cost.Innovations Driving the C-arms Market Growth
Rapid advancement in technology are expected to impact the landscape of the C-arms market positively. In November 2023, a United States-based company providing innovative solutions for orthopedic imaging, Orthoscan Inc., showcased a 32-inch 4K display monitor for its mini-C-arm at the annual conference meeting Radiological Society of North America (RSNA) 2023. The touchscreen monitor will provide powerful imaging capabilities to its TAU2020 mini-C-arm device, facilitating a quick and accurate diagnosis of the patients.Global C-arms Market Segmentation
C-arms Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Type
- Mobile C-Arms
- Fixed C-Arms
Market Breakup by Technology
- Image Intensifiers
- Flat Panel
Market Breakup by Detector
- Flat Panel Detector
- Image Intensifier
Market Breakup by Application
- Orthopedic and Trauma Surgeries
- Cardiovascular Surgeries
- Neuro Surgeries
- Gastrointestinal Surgeries
- Urology
- Pain Management
- General Surgery
- Others
Market Breakup by End User
- Hospitals
- Clinical Research Institutes
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global C-arms Market Regional Analysis
North America accounts for the largest share of the C-arms market which can be attributed to the increasing prevalence of chronic diseases such as cardiovascular diseases which require surgical intervention. According to the data released by the Centers for Disease Control and Prevention, cardiovascular diseases stand as the leading cause of death in the United States, with one person dying every 33 seconds from the medical condition. Further, it is estimated that 805,000 people suffer from a heart attack in the United States every year and the number is expected to rise with the growing geriatric population. Since the C-arms offer robust intraoperative cardiovascular imaging, their demand is expected to increase significantly in the region.Asia Pacific also holds a significant C-arms market value and is anticipated to witness rapid growth in the coming years. The major key drivers are the huge population size and the rising disease burden. Further, technological advancement and intensive research and development activities to improve the healthcare system are expected to accelerate the market growth.
Global C-arms Market: Competitor Landscape
In October 2023, a German MedTech company, Siemens Healthineers launched a Startup Accelerator Program in collaboration with Nasscom Center of Excellence (CoE) and partner with emerging startups such as Tagbox and Imaginate. The program is designed to emphasize on multiple domains, with radiology imaging, advanced image guided therapy, in-vitro diagnostics, and digitally enabled services as a key area of attention. The utilization of digital technology solutions increased lead conversion for the company's C-arm products and helped save 25% of the expenditure on in-person sales meetings and training. Thus, the integration of digital solutions into existing systems will help in the expansion of the C-arms market size in the coming years.The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:
- Canon Inc.
- Koninklijke Philips N.V.
- Ziehm Imaging
- Siemens Healthcare GmbH
- MS WESTFALIA GMBH
- STEPHANIX
- Villa Sistemi Medicali Spa.
- Hologic, Inc.
- ITALRAY
- Shimadzu Corporation
- Eurocolumbus s.r.l.
- AADCO Medical, Inc.
- BMI Biomedical International s.r.l.
- Assing S.p.A
- Abbott
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Canon Inc.
- Koninklijke Philips N.V.
- Ziehm Imaging
- Siemens Healthcare GmbH.
- MS WESTFALIA GMBH
- STEPHANIX
- Villa Sistemi Medicali Spa.
- Hologic, Inc.
- ITALRAY
- Shimadzu Corporation
- Eurocolumbus s.r.l.
- AADCO Medical, Inc.
- BMI Biomedical International s.r.l.
- Assing S.p.A
- Abbott.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
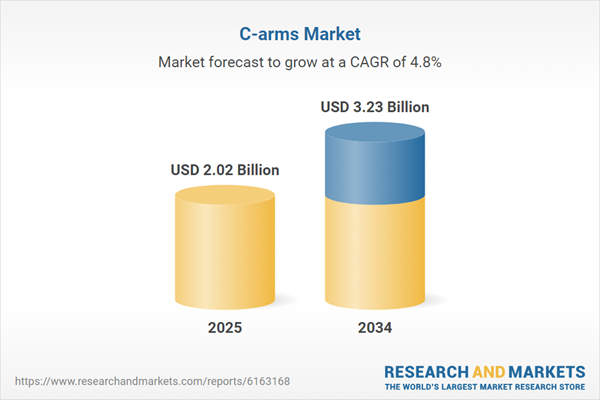
| Estimated Market Value ( USD | $ 2.02 Billion |
| Forecasted Market Value ( USD | $ 3.23 Billion |
| Compound Annual Growth Rate | 4.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |