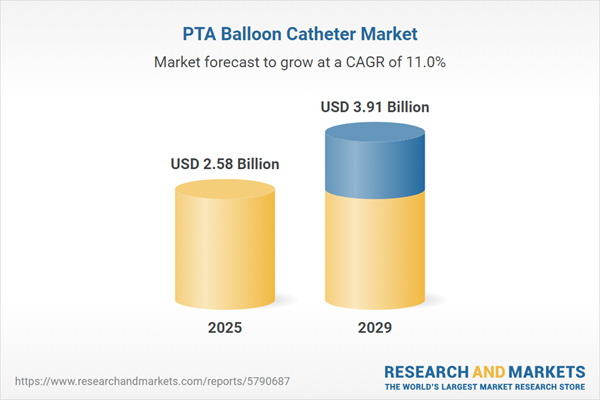
The PTA balloon catheter market size is expected to see rapid growth in the next few years. It will grow to $3.91 billion in 2029 at a compound annual growth rate (CAGR) of 11%. The growth in the forecast period can be attributed to rising cardiovascular disease cases, aging population dynamics, focus on minimally invasive procedures, increasing diabetes and obesity rates, healthcare infrastructure expansion. Major trends in the forecast period include enhanced training and education, shift to value-based healthcare, focus on safety and biocompatibility, robotics and navigation systems, patient-specific treatment.
The rising prevalence of cardiovascular disorders is anticipated to drive the growth of the PTA balloon catheter market in the coming years. Cardiovascular disorders encompass conditions that affect the heart or blood vessels, and an increase in these diseases necessitates advanced diagnostic and treatment options, such as PTA balloon catheters, thus boosting market demand. For example, in May 2023, a report by The Indiana Department of Health (IDOH) indicated that out of an estimated 250,000 central line-associated bloodstream infections (CLABSIs) occurring annually in U.S. hospitals, around 80,000 are reported in intensive care units (ICUs). Consequently, the growing prevalence of cardiovascular disorders is supporting the expansion of the PTA balloon catheter market.
The growing geriatric population is anticipated to drive the expansion of the PTA balloon catheter market in the coming years. The geriatric population, comprising individuals aged 65 and older, has unique healthcare needs related to aging. PTA balloon catheters play a vital role in managing cardiovascular conditions in elderly individuals, supporting vascular health and overall well-being. For example, in October 2022, the World Health Organization, a specialized health agency, reported that by 2030, 1 in 6 individuals globally will be aged 60 or older, with this figure projected to reach 2.1 billion by 2050. Thus, the growing geriatric population is fueling the growth of the PTA balloon catheter market.
Leading companies in the PTA balloon catheter market are prioritizing innovative products like infusion systems to improve patient outcomes and enhance procedural efficiency. Infusion systems are medical devices specifically designed for the intravenous administration of fluids, medications, or nutrients to patients. They provide precise control over dosage and flow rates, thereby improving treatment effectiveness and ensuring patient safety. For example, in April 2022, Terumo Corporation, a Japan-based medical device company, launched Terumo Medical Care Solutions (TMCS). This initiative represents a significant advancement in Terumo's commitment to positively impacting society through healthcare. By emphasizing quality time for both healthcare providers and patients, TMCS seeks to tackle contemporary challenges in medical care while enhancing the overall patient experience. This move not only underscores Terumo's century-long legacy in medical technology but also strategically positions the company for future growth in an increasingly complex healthcare landscape.
Major companies in the PTA balloon catheter market are strategically focusing on product launches, such as the PTA balloon catheter, to serve as a unique bridge between renal and vascular divisions. A notable example is Nipro Corporation, a Japan-based medical equipment manufacturing company. In March 2022, Nipro Corporation introduced the Cronus HP PTA balloon catheter, which offers robust performance at high pressures, graduated control angioplasty using a single balloon, and enhanced ease of entry across lesions to minimize balloon dog boning. These features provide an innovative edge, addressing procedural challenges and potentially improving overall efficacy, making it a valuable tool for vascular surgeons and interventionalists.
In December 2022, Boston Scientific Corporation, a US-based manufacturing company, acquired Acotec Scientific Holdings Ltd. for an undisclosed sum. This acquisition is intended to bolster Boston Scientific Corporation's product portfolio and broaden its market presence within the medical device sector. Acotec Scientific Holdings Ltd. is a China-based medical technology company that specializes in the development and manufacturing of innovative medical devices, particularly in the realm of interventional cardiology and related fields.
Major companies operating in the PTA balloon catheter market include Abbott Laboratories, Medtronic PLC, Boston Scientific Corporation, Lepu Medical, B. Braun Corporation, Terumo, Nipro Medical, Teleflex, Cook Medical, Biotronik, Merit Medical, Microport Scientific, Cardiovascular Systems, Well Lead Medical, Cardionovum, Surmodics, JOTEC, Creagh Medical, Natec Medical, TriReme Medical, Andratec, Acotec Scientific, Kaneka Corporation.
North America was the largest region in the PTA balloon catheter market share in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the pta balloon catheter market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the pta balloon catheter market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
A PTA balloon catheter is a medical procedure designed to widen a narrowed vessel opening using a small, flexible plastic tube or catheter with a 'balloon' at its end. This technique is utilized in minimally invasive catheterization procedures.
The primary types of PTA balloon catheters include polyurethane and nylon. Polyurethane is a material derived from plastic in various forms, with applications spanning coronary artery disease and peripheral vascular disease. These catheters find use in various healthcare settings, including hospitals, ambulatory surgical centers, and others.
The PTA balloon catheters market research report is one of a series of new reports that provides PTA balloon catheters market statistics, including the PTA balloon catheters industry’s global market size, regional shares, competitors with a PTA balloon catheters market share, detailed PTA balloon catheters market segments, market trends and opportunities, and any further data you may need to thrive in the PTA balloon catheters industry. This PTA balloon catheters market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The PTA balloon catheter market consists of sales of non-compliant (high-pressure) balloons, semi-compliant (mid pressure), and compliant (elastomeric) balloons. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
PTA Balloon Catheter Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on pta balloon catheter market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for pta balloon catheter? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The pta balloon catheter market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Polyurethane; Nylon2) By Application: Coronary Artery Disease; Peripheral Vascular Disease
3) By End User: Hospitals; Ambulatory Surgical Centers; Other End Users
Subsegments:
1) By Polyurethane: Non-compliant Balloons; Compliant Balloons2) By Nylon: Non-compliant Balloons; Compliant Balloons
Key Companies Mentioned: Abbott Laboratories; Medtronic PLC; Boston Scientific Corporation; Lepu Medical; B. Braun Corporation
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Abbott Laboratories
- Medtronic PLC
- Boston Scientific Corporation
- Lepu Medical
- B. Braun Corporation
- Terumo
- Nipro Medical
- Teleflex
- Cook Medical
- Biotronik
- Merit Medical
- Microport Scientific
- Cardiovascular Systems
- Well Lead Medical
- Cardionovum
- Surmodics
- JOTEC
- Creagh Medical
- Natec Medical
- TriReme Medical
- Andratec
- Acotec Scientific
- Kaneka Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 175 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.58 Billion |
| Forecasted Market Value ( USD | $ 3.91 Billion |
| Compound Annual Growth Rate | 11.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |