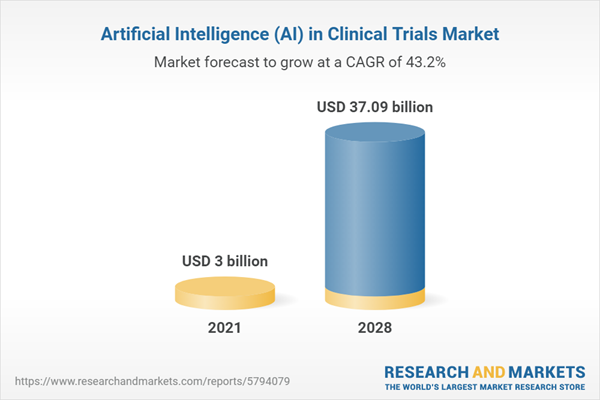
AI In clinical trials market is expected to grow at a CAGR of 43.21% from a market size of US$3.003 billion in 2021 to reach US$37.093 billion in 2028.
AI in clinical trials refers to the application of artificial intelligence solutions and tools in clinical trials and drug discovery procedures including planning the patient recruitment and monitoring systems, designing the trial plan, and selecting the trial site. The adoption of AI technology in clinical trials helps in overcoming the limitations of conventional clinical trial procedures by generating results at a faster pace and enhances the diversity of the population used in a clinical trial. By using AI technology at the initial stages of a clinical trial, a pharmaceutical firm can create a sound environment to increase the probability of success of a clinical trial thereby preventing the financial loss that could be incurred by the firm in case of a failure. Hence, the increasing adoption of AI technology across all healthcare solutions is likely to generate greater demand for the application of AI in the clinical trials market.Market Drivers
The growing demand for drug development to fight rare and genetic diseases
The research and development in the genetic and oncological drug development division offer the opportunity to incorporate AI tools and technology to innovate effective drugs to target such diseases. Recent developments in the genetic context and research of certain rare diseases provide greater chances for utilizing AI-based clinical trials to accelerate the research process to identify the source of origin of a particular disease and design a trial plan to investigate the effectiveness of a potential medicine. For instance, Illumina Inc. revealed that approximately 80% of rare diseases diagnosed are linked to a genetic source. Therefore, AI-assisted clinical trial procedures could help in thoroughly analyzing existing information to create breakthroughs to generate effective drugs against the rare occurrence of such diseases.Rigorous labor involved in conducting clinical trials
The discovery and generation of new pharmaceutical drugs is an extremely time-consuming process that requires extensive labor and resources to produce results. At a clinical trial, a medicine has to go through various phases and testing activities which generates a vast amount of data that could be tedious and labor-intensive for research analysts to go through all the data produced. The integration of AI technology and big data analytics aids in the automated and faster processing of such data to derive meaningful observations which can be automatically communicated to all the researchers involved in the clinical trials. Therefore, the extensive labor required to conduct a clinical trial and the possibility of generating drugs for rare and genetic diseases is the key factor driving the demand for AI in the clinical trials market.The unavailability and bias of health data in clinical trials can slow down the advancement of AI in the clinical trials market.
The working of AI technology in clinical trials requires the analysis of large amounts of pre-existing datasets to derive significant insights to be helpful in the progression of clinical trials. The datasets that currently exist might not be sufficient to develop drugs for any newly emerged or unknown diseases such as the Corona Virus. Since the behavior patterns and anatomy of Corona Virus were unknown, it was initially difficult for researchers to develop an effective antibody to fight the virus. In such circumstances where past data cannot be relied upon, the effectiveness of AI-based solutions could be limited. In addition to this, the presence of bias in any of the reference datasets could lead to biased insights and results in AI-sponsored clinical trials. For instance, most of the datasets and databases used for genetic research and trials are primarily Europe-centric. Therefore, the results derived from such samples cannot be generalized to the people of all geographical regions.Market Developments
In March 2023, Aloha Health Network and Leal Health Companies launched an integrated AI-powered SaaS platform to assist pharmaceutical and biotechnological companies in accelerating patent recruitment procedures for clinical trials.In March 2023, Paige and Mindpeak, two biotechnological companies specializing in the application of AI software collaborated to launch a developed Paige Platform that amalgamates the AI-powered services of both companies to upgrade the accuracy and reliability of cancer diagnoses.
In February 2023, Lantern Pharma Inc. introduces new features to its RADR platform which combines AI technology with ML models to assist in the discovery of effective oncological drugs made using antibody-drug conjugates.
North America holds high potential in the AI in clinical trials market and is expected to grow significantly during the forecasted period.
North America has high levels of healthcare spending, which has driven the demand for more efficient and effective healthcare solutions and pharmaceutical drugs. As a result of this, leading biotechnological companies are actively investing in the production of better technological equipment to assist in pharmacological processes including drug discovery and clinical trial processes conducted by pharmaceutical companies. According to the World Health Organization, in 2021, the USA is leading in the clinical trial field and has registered approximately 157,618 clinical trials over the last two decades. In addition, the emergence of leading AI-powered clinical start-up companies in the US such as Owkin, Deep Lens, Unlearn.AI, VeriSIM Life, and Aicure provide greater opportunities for widening the application of AI technology in clinical trial procedures.Apart from these newly formed companies, leading pharmaceutical drug manufacturing companies in the US such as Johnson&Johnson, Pfizer, and Bristol Myers Squibb are either collaborating with innovative AI tech companies or investing money to create their own AI-powered tools to be used in clinical trials. Hence, the considerable number of clinical trials and the increasing adoption of AI technology by pharmaceutical companies in the US are expected to contribute to the growth of AI-empowered clinical trials in North America.
Market Segmentation:
By Process
- Trial Design
- Patient Selection
- Site Selection
- Patient Monitoring
By Application
- Biosensors
- Smartphone Applications
- Wearables
By Geography
- North America
- USA
- Canada
- Mexico
- South America
- Brazil
- Argentina
- Others
- Europe
- United Kingdom
- Germany
- France
- Italy
- Spain
- Others
- Middle East and Africa
- Saudi Arabia
- UAE
- Others
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Vietnam
- Others
Table of Contents
Companies Mentioned
- ConcertAI
- Saama Technologies LLC
- PathAI
- Owkin Inc.
- Aitia
- Neuroute
- AiCure
- Unlearn AI
- VeriSIM Life
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 146 |
| Published | May 2023 |
| Forecast Period | 2021 - 2028 |
| Estimated Market Value ( USD | $ 3 billion |
| Forecasted Market Value ( USD | $ 37.09 billion |
| Compound Annual Growth Rate | 43.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 9 |