External Defibrillator Introduction
External defibrillators are medical devices used to deliver an electrical shock to the heart in order to restore its normal rhythm in cases of cardiac arrest. They are widely used in hospitals, clinics, and emergency medical services (EMS) to treat patients experiencing sudden cardiac arrest.There are two types of external defibrillators: automated external defibrillators (AEDs) and manual external defibrillators. AEDs are designed for use by non-medical personnel and are used in public places, while manual external defibrillators are used in hospitals and clinics by trained medical professionals.
The market for external defibrillators has seen significant growth in recent years due to the increasing incidence of cardiovascular diseases, aging population, and technological advancements in defibrillator devices. The growing demand for automated external defibrillators (AEDs) in public places such as airports, railway stations, and shopping malls is also contributing to the growth of the market.
The market is highly competitive, with several major players operating in the market. These players are focusing on product innovations, strategic partnerships, and acquisitions to maintain their market position and expand their product portfolios. In addition, the market is also witnessing a trend towards the development of wearable defibrillator devices, which can be used by patients at home.
Overall, the external defibrillator market is expected to continue to grow due to the increasing prevalence of cardiovascular diseases and the growing demand for AEDs in public places.
External Defibrillator Market Scenario
External defibrillators are medical devices used to deliver an electrical shock to the heart in order to restore its normal rhythm in cases of life-threatening cardiac arrhythmias. These devices are used in emergency settings such as hospitals, ambulances, and other healthcare facilities, as well as by first responders such as police and fire departments.The global market for external defibrillators is primarily driven by an increasing incidence of sudden cardiac arrest (SCA) and the growing awareness about the importance of defibrillation during the golden hour to improve patient outcomes. Furthermore, the rising adoption of public access defibrillators (PADs) in public places and increasing government initiatives to support the use of these devices are also contributing to the growth of the market.
However, factors such as high cost of devices, stringent regulatory policies, and the need for regular maintenance and training of personnel to operate these devices are likely to restrain the market growth to some extent. Nevertheless, the introduction of technologically advanced and portable devices, increasing adoption of smart wearable defibrillators, and rising demand from emerging markets are expected to provide significant growth opportunities for the market in the coming years.
External Defibrillator Market Segmentations
Market Breakup by Product
- Wearable Cardioverter Defibrillators
- Implantable Cardioverter Defibrillators
- Manual External Defibrillators
Automated External Defibrillators
- Semi-Automated ED
- Fully Automated ED
Market Breakup by End User
- Pre-Hospital
- Public Access Market
- Hospital
- Home Healthcare
- Alternate Care Market
- Others
Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Key Trends in the External Defibrillator Market
Some key trends in the external defibrillator market are:- Increasing demand for automated external defibrillators (AEDs) for public access locations such as airports, schools, and stadiums
- The emergence of wearable defibrillators that can be worn by patients at risk of sudden cardiac arrest
- Integration of advanced technologies such as artificial intelligence and cloud-based connectivity for real-time monitoring and data analysis
- Growing popularity of defibrillator rental and leasing services to provide cost-effective solutions for hospitals and clinics
- Increasing adoption of defibrillator training programs for first responders, healthcare professionals, and laypeople to improve the chances of survival from cardiac arrest
External Defibrillator Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Koninklijke Philips N.V
- Stryker Corporation
- ZOLL Medical Corporation
- Nihon Kohden Corporation
- Boston Scientific Corporation
- Schiller AG
- MS Westfalia GmbH
- AMI Italia
- Bexen Cardio
- Silverline Meditech Pvt. Ltd
- Mediana Co., Ltd
- Shenzhen Mindray Bio-Medical Electronics
- CU Medical Germany GmbH
- BPL Medical Technologies
- Dixion Vetrieb medizinischer Geräte GmbH
- Bioevopeak Co., Ltd.
Table of Contents
Companies Mentioned
- Koninklijke Philips N.V.
- Stryker Corporation
- Zoll Medical Corporation
- Nihon Kohden Corporation
- Boston Scientific Corporation
- Progettisrl
- Schiller AG
- Ms Westfalia GmbH
- Ami Italia
- Bexen Cardio
- Silverline Meditech Pvt. Ltd.
- Mediana Co. Ltd.
- Shenzhen Mindray Bio-Medical Electronics
- Cu Medical Germany GmbH
- Bpl Medical Technologies
- Dixion Vetrieb Medizinischer Geräte GmbH
- Bioevopeak Co. Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | May 2023 |
| Forecast Period | 2023 - 2031 |
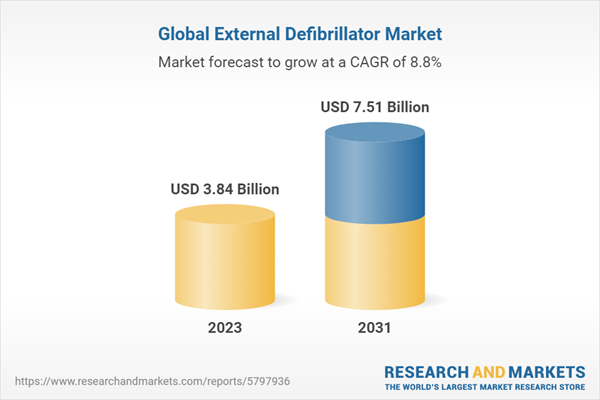
| Estimated Market Value ( USD | $ 3.84 Billion |
| Forecasted Market Value ( USD | $ 7.51 Billion |
| Compound Annual Growth Rate | 8.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 17 |