Medical Device Outsourcing Market: Introduction
Medical device outsourcing refers to the process where medical device manufacturers or healthcare providers contract with third-party companies or external organizations to perform specific tasks or services related to the development, production, testing, or marketing of medical devices. This practice allows companies to focus on their core competencies, while leveraging the expertise of specialized partners to accomplish various tasks more efficiently and cost-effectively.Some of the benefits of medical device outsourcing include:
- Cost savings: Outsourcing can help companies reduce overall costs by leveraging the expertise of specialized partners, who can often perform tasks more efficiently and at a lower cost than in-house resources
- Access to expertise: By outsourcing specific tasks or services, companies can gain access to specialized knowledge and skill sets that may not be available in-house, enabling them to develop and produce high-quality devices more effectively
- Improved focus on core competencies: Outsourcing non-core functions allows companies to focus on their primary strengths and competencies, leading to increased efficiency and better allocation of resources
- Faster time to market: Outsourcing can help companies bring their products to market more quickly by streamlining the development and manufacturing process, leveraging the expertise of partners who have proven track records in their respective fields
- Risk mitigation: Outsourcing can help companies manage and mitigate risks associated with the development and manufacturing of medical devices by distributing responsibilities and liabilities among multiple partners
- Scalability and flexibility: Outsourcing allows companies to scale their operations up or down as needed, based on market demand and other factors, without the need for significant investments in infrastructure or personnel
- Compliance and regulatory support: Outsourcing partners with experience in the medical device industry can help companies navigate complex regulatory requirements and ensure that their products meet all necessary standards and guidelines
- Enhanced innovation: By working with specialized partners, companies can benefit from their unique insights and innovative approaches, leading to the development of more advanced and effective medical devices
Medical Device Outsourcing Market Segmentations
The market can be categorised into segments, product type, device type, applications, and region.Market Breakup by Services
- Quality Assurance
- Regulatory Affairs Services
- Product Design and Development Services
- Product Testing and Sterilization Services
- Product Implementation Services
- Product Upgrade Services
- Product Maintenance Services
- Raw Material Services
- Medical Electrical Equipment Services
- Contract Manufacturing
- Materials and Chemical Characterization
- Others
Market Breakup by Product Type
- Finished Goods
- Electronics
- Raw Materials
Market Breakup by Device Type
- Class-I
- Class -II
- Class -III
- Cardiology
- Diagnostic Imaging
- Orthopaedic
- IVD
- Ophthalmic General
- Plastic Surgery
- Drug Delivery
- Dental
- Endoscopy
- Diabetes Care
- Others
Market Breakup by End User
- Small Medical Device Company
- Medium Medical Device Company
- Large Medical Device Company
- Others
Medical Device Outsourcing Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Medical Device Outsourcing Market Scenario
The medical device outsourcing market has experienced significant growth in recent years due to the increasing demand for advanced medical devices, rising healthcare costs, and the need for companies to focus on their core competencies. This market expansion is fuelled by the complex regulatory environment, rapid technological advancements, and a growing emphasis on cost-efficiency, which drive medical device manufacturers to seek specialized expertise from external partners.As a result, the market has seen a surge in service providers offering a wide range of services, such as product design and development, manufacturing, testing, regulatory compliance, and supply chain management. The global market scenario for medical device outsourcing continues to evolve, with emerging markets becoming increasingly attractive destinations for outsourcing due to lower labour costs and the availability of skilled professionals.
Key Players in the Global Medical Device Outsourcing Market
The report gives an in-depth analysis of the key players involved in the medical device outsourcing market, sponsors manufacturing the drugs, and putting them through trials to get FDA approvals. The companies included in the market are as follows:- SGS SA
- TOXIKON
- Pace Analytical
- Intertek Group plc
- WuXi AppTec
- North American Science Associates, Inc
- American Preclinical Services, LLC
- Sterigenics
- Charles River Laboratories
- Celestica Inc
- Creganna
- FLEX LTD
- Heraeus Holding
- Integer Holdings Corporation
- Nortech Systems, Inc
- Plexus Corp
- Sanmina Corporation
- EUROFINS SCIENTIFIC
- TE Connectivity, ICON plc
- Parexel International Corporation
- Labcorp Drug Development
- Tecomet, Inc
- IQVIA
- Syneos Health
- PROVIDIEN LLC.
Table of Contents
Companies Mentioned
- Sgs Sa
- Toxikon
- Pace Analytical
- Intertek Group plc.
- Wuxi Apptec
- North American Science Associates, Inc.
- American Preclinical Services, LLC.
- Sterigenics
- Charles River Laboratories
- Celestica Inc.
- Creganna
- Flex Ltd.
- Heraeus Holding
- Integer Holdings Corporation
- Nortech Systems, Inc.
- Plexus Corp.
- Sanmina Corporation
- Eurofins Scientific
- Te Connectivity, Icon plc
- Parexel International Corporation
- Labcorp Drug Development
- Tecomet, Inc.
- Iqvia
- Syneos Health
- Providien LLC.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | May 2023 |
| Forecast Period | 2023 - 2031 |
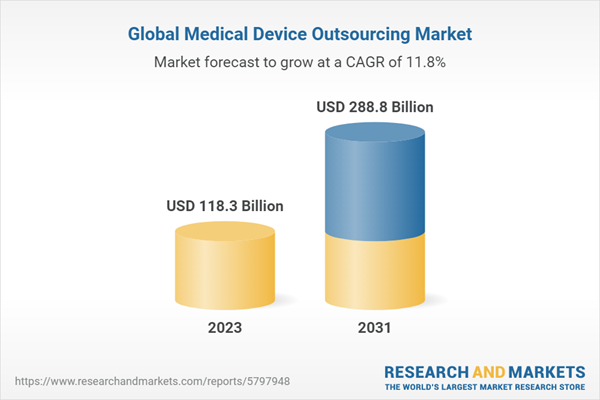
| Estimated Market Value ( USD | $ 118.3 Billion |
| Forecasted Market Value ( USD | $ 288.8 Billion |
| Compound Annual Growth Rate | 11.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |