Influenza Vaccine: Introduction
Influenza vaccines, also known as flu shots, are formulated to protect against the influenza virus, a contagious respiratory illness with varying severity levels. Influenza vaccines are updated annually, as the virus mutates rapidly, and different strains circulate each flu season. The vaccines aim to stimulate an immune response by using inactivated or weakened viruses, or specific viral components like recombinant proteins or viral vectors, to mimic the natural infection.Flu vaccines are usually trivalent or quadrivalent, meaning they protect against three or four strains of the virus, respectively. The World Health Organization (WHO) recommends the strains to be included in the vaccine each year, based on global surveillance data and predictions about the most prevalent strains for the upcoming flu season.
Influenza vaccination is recommended for most individuals, particularly those at higher risk of developing severe complications, such as the elderly, young children, pregnant women, and individuals with chronic health conditions. The vaccines are typically administered via an injection in the upper arm, but nasal spray vaccines are also available for specific age groups. While flu vaccines do not guarantee complete protection from the virus, they significantly reduce the risk of infection and minimize the severity of illness for those who contract the flu. In addition, widespread vaccination contributes to herd immunity, helping to protect vulnerable populations who may not be able to receive the vaccine themselves.
Influenza Vaccine Market Scenario
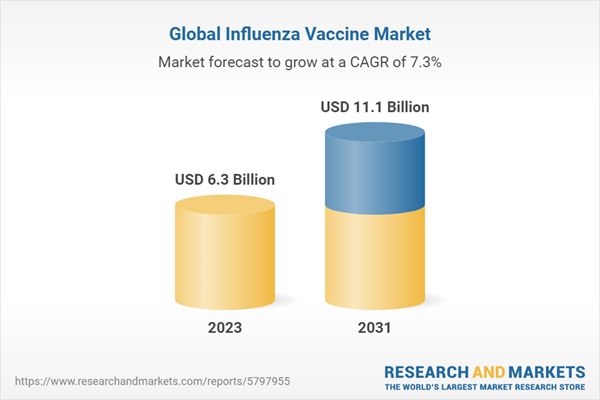
The global influenza vaccine market is witnessing significant growth as a result of increased awareness about the health risks associated with influenza and the importance of vaccination in preventing flu-related complications. Influenza, or the flu, is a highly contagious respiratory illness caused by the influenza virus, with varying degrees of severity. Annual vaccination is recommended as the virus mutates rapidly, leading to different strains circulating each flu season.Influenza vaccines aim to stimulate an immune response using inactivated or weakened viruses or specific viral components to mimic a natural infection. These vaccines are typically trivalent or quadrivalent, meaning they protect against three or four strains of the virus, respectively. The World Health Organization (WHO) recommends the strains to be included in the vaccines each year based on global surveillance data and predictions about the most prevalent strains for the upcoming flu season.
The market's growth is driven by factors such as expanding target populations for vaccination, technological advancements in vaccine development, and increasing investments in research and development.
North America: The largest market for influenza vaccines, driven by factors such as advanced healthcare infrastructure, high vaccination rates, and significant investments in research and development.
Europe: The second-largest market, with increasing demand for flu vaccines due to a growing aging population, a supportive regulatory framework, and an expanding healthcare sector.
Asia-Pacific: Expected to witness the highest growth rate due to factors such as a rapidly growing population, increasing awareness of influenza and vaccination, and growing investments in healthcare infrastructure and research.
Influenza Vaccine Market Segmentations
Market Breakup by Vaccine Type
- Quadrivalent
- Trivalent
Market Breakup by Technology
- Egg Based
- Cell Based
Market Breakup by Age Group
- Pediatric
- Adult
Market Breakup by Route of Administration
- Injection
- Nasal Spray
Market Breakup by Distribution Channel
- Hospital Pharmacy
- Government and Institution Supply
- Others
Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Key Trends in the Influenza Vaccine Market
Some key trends of the market are as follows:- Increasing awareness of influenza and the importance of vaccination: The rising awareness of the health risks associated with influenza and the significance of vaccination in preventing flu-related complications has led to increased demand for flu vaccines
- Expanding target populations for vaccination: Expanded vaccination recommendations, including wider age groups and high-risk populations, have contributed to the growth of the influenza vaccine market
- Technological advancements in vaccine development: Innovations in vaccine development, such as cell-based and recombinant technologies, have improved the efficacy and production efficiency of influenza vaccines, further driving market growth
Influenza Vaccine: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- AstraZeneca plc
- Biodiem Limited
- CSL Limited
- Emergent BioSolutions Inc
- F. Hoffmann-La Roche AG
- Gamma Vaccines Pty Ltd
- GlaxoSmithKline Plc
- Merck and Co., Inc
- Pfizer Inc
- Sanofi S.A.
Table of Contents
Companies Mentioned
- Astrazeneca plc
- Biodiem Limited
- Csl Limited
- Emergent Biosolutions Inc.
- F. Hoffmann-La Roche AG
- Gamma Vaccines Pty Ltd
- GlaxoSmithKline plc.
- Merck and Co. Inc.
- Pfizer Inc.
- Sanofi S.A.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 147 |
| Published | May 2023 |
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 6.3 Billion |
| Forecasted Market Value ( USD | $ 11.1 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |