Cholera Vaccines Market: Introduction
Cholera vaccines are biological preparations that provide protection against cholera, a severe diarrheal disease caused by the bacterium Vibrio cholerae. The disease is transmitted through contaminated water and food, and can result in rapid dehydration, severe electrolyte imbalance, and even death if left untreated. Cholera is particularly prevalent in regions with poor sanitation, inadequate water treatment, and limited access to healthcare facilities. Vaccination is an essential tool in preventing cholera outbreaks and managing the disease in endemic regions.Uses of Cholera Vaccines:
- Preventive measure: Cholera vaccines are administered to individuals in areas where cholera is endemic, or where there is a risk of an outbreak, to prevent the spread of the disease
- Travelers: Travelers to cholera-affected regions may be advised to receive the cholera vaccine to protect themselves from infection during their stay
- Emergency response: Cholera vaccines can be used during humanitarian crises, such as natural disasters or refugee situations, where there is an increased risk of cholera outbreaks due to the disruption of water and sanitation systems
1. Disease prevention: Cholera vaccines significantly reduce the risk of infection, helping to prevent illness and the spread of the disease within communities.
2. Reduced morbidity and mortality: By preventing cholera infection, the vaccines help decrease the number of severe cases and fatalities associated with the disease.
3. Cost-effectiveness: Cholera vaccination campaigns can be more cost-effective than treating individual cases of the disease, as they help prevent the need for expensive medical interventions, such as intravenous rehydration therapy and hospitalization.
4. Strengthening public health efforts: Cholera vaccines are an important component of comprehensive public health strategies to control the disease, which also includes improving water and sanitation systems and promoting hygiene practices.
5. Support for healthcare systems: By reducing the number of cholera cases, vaccines can help ease the burden on healthcare systems in affected regions, allowing them to allocate resources more effectively.
There are currently several cholera vaccines available, including oral vaccines like Dukoral, Shanchol, and Euvichol. These vaccines typically require multiple doses to provide optimal protection, and the immunity they confer may decrease over time, necessitating periodic booster doses. It is important to note that while cholera vaccines are highly beneficial, they should not replace efforts to improve water and sanitation infrastructure, as well as promote proper hygiene practices to control the disease in the long term.
cholera Vaccines Market Segmentations
The market can be categorised into product, end user, and region.Cholera Vaccines Market Breakup by Product
- Dukoral
- Shanchol
- Vaxchora
- Euvichol
- Others
Cholera Vaccines Market Breakup by End User
- Hospitals & Clinics
- Research and Academic Laboratories
- Others
Cholera Vaccines Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Cholera Vaccines Market Scenario
The cholera vaccines market has been experiencing steady growth, driven by several factors that contribute to the increasing demand for effective prevention measures against cholera. The market is primarily influenced by the prevalence of cholera in regions with poor sanitation, inadequate water treatment, and limited access to healthcare facilities, which increases the need for vaccination campaigns.- One of the primary drivers of the cholera vaccines market is the increased focus on public health initiatives by governments, non-governmental organizations, and international agencies such as the World Health Organization (WHO). These organizations have been actively engaged in implementing cholera vaccination programs in regions where the disease is endemic or at risk of outbreaks. This has led to a higher demand for cholera vaccines, as well as increased funding for their development and distribution
- Another factor contributing to the growth of the cholera vaccines market is the rise in global travel, which exposes more individuals to cholera-affected regions. As a result, the demand for cholera vaccines among travellers has increased, leading to a wider distribution of the vaccines in travel clinics and other healthcare facilities
- Climate change and its effects on weather patterns have also contributed to the increasing incidence of cholera outbreaks in some regions. Flooding, for example, can disrupt water and sanitation systems, leading to the contamination of water supplies and the spread of cholera. This has heightened the need for effective cholera vaccines to protect vulnerable populations and prevent the spread of the disease
- The cholera vaccines market is segmented based on product types (such as Dukoral, Shanchol, and Euvichol), distribution channels (public healthcare providers, private healthcare providers, and travel clinics), and geographical regions (North America, Europe, Asia-Pacific, and the rest of the world). The Asia-Pacific region, in particular, has been a significant market for cholera vaccines, given the higher prevalence of the disease in certain countries within the region
- The market is characterized by the presence of several key players, including both established pharmaceutical companies and emerging biotechnology firms, involved in the development, manufacturing, and distribution of cholera vaccines. As the demand for effective cholera vaccines continues to grow, these companies are likely to invest in research and development to improve existing vaccines and create new, more effective products
Key Players in the Global Cholera Vaccines Market
The report gives an in-depth analysis of the key players involved in the cholera vaccines market, sponsors manufacturing the drugs, and putting them through trials to get FDA approvals. The companies included in the market are as follows:- Sanofi-aventis Groupe
- Emergent BioSolutions Inc
- Valneva SE
- GlaxoSmithKline plc
- Intervet Inc
- EuBiologics Co., Ltd
- Astellas Pharma Inc
- Celldex Therapeutics
- Johnson & Johnson Services Inc
- Merck & Co. Inc.
Table of Contents
Companies Mentioned
- Sanofi-Aventis Groupe
- Emergent Biosolutions Inc.
- Valneva Se
- GlaxoSmithKline plc.
- Intervet Inc.
- Eubiologics Co. Ltd.
- Astellas Pharma Inc.
- Celldex Therapeutics.
- Johnson & Johnson Services Inc.
- Merck & Co. Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 147 |
| Published | April 2023 |
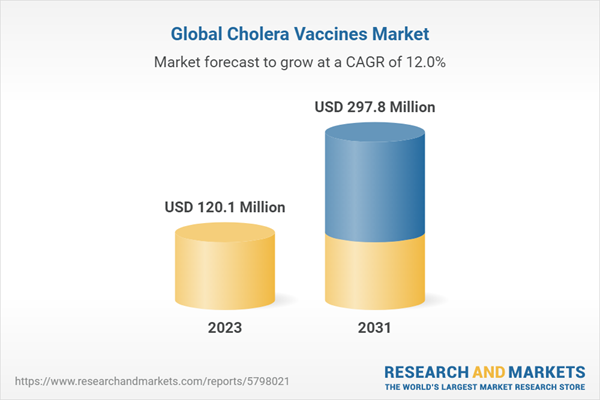
| Forecast Period | 2023 - 2031 |
| Estimated Market Value ( USD | $ 120.1 Million |
| Forecasted Market Value ( USD | $ 297.8 Million |
| Compound Annual Growth Rate | 12.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |