Introduction
Autoinjectors are medical devices designed to deliver a precise dose of medication through a pre-filled syringe. They are self-administered by patients or caregivers and are commonly used for the treatment of chronic conditions such as diabetes, multiple sclerosis, rheumatoid arthritis, and allergies. Autoinjectors provide convenience, ease of use, and increased safety in medication delivery, making them a preferred choice for many patients.Key Trends in the Autoinjector Market
Some key trends involved in the autoinjector market are as follows:- Growing Prevalence of Chronic Diseases: The increasing prevalence of chronic diseases, such as diabetes and autoimmune disorders, is driving the demand for self-administered treatments. Autoinjectors offer a convenient and efficient way for patients to administer their medications at home, reducing the need for frequent hospital visits
- Technological Advancements: Continuous advancements in autoinjector technology have improved usability, safety, and patient experience. Manufacturers are focusing on developing devices with features like electronic dose monitoring, voice guidance, and connectivity with mobile apps for enhanced adherence and monitoring
- Shift towards Biologics and Biosimilars: The rise in the use of biologic drugs and biosimilars has created a need for reliable and user-friendly administration devices. Autoinjectors are preferred for these medications due to their ability to deliver precise doses and accommodate the high viscosity of biologics
- Increasing Patient Preference for Self-Administration: Patients are increasingly seeking self-administration options to have more control over their treatment and improve their quality of life. Autoinjectors empower patients to manage their conditions independently, promoting self-care and reducing reliance on healthcare professionals
Autoinjector Market Segmentations
Market Breakup by Type
- Disposable
- Reusable
Market Breakup by Application
- Rheumatoid Arthritis
- Multiple Sclerosis
- Anaphylaxis
- Others
Market Breakup by Route of Administration
- Subcutaneous
- Intramuscular
Market Breakup by End User
- Hospitals
- Clinics
- Home Care Settings
- Others
Market Breakup by Region
North America
- United States of America
- Canada
Europe
- United Kingdom
- Germany
- France
- Italy
- Others
Asia Pacific
- China
- Japan
- India
- ASEAN
- Australia
- Others
Latin America
- Brazil
- Argentina
- Mexico
- Others
Middle East and Africa
- Saudi Arabia
- United Arab Emirates
- Nigeria
- South Africa
- Others
Autoinjector Market Scenario
The autoinjector market is a rapidly growing segment within the healthcare industry, driven by the increasing demand for self-administered treatments and the need for convenient and reliable drug delivery devices. Autoinjectors are designed to provide patients with an efficient and user-friendly method of administering medication at home, eliminating the need for frequent visits to healthcare facilities.One of the key factors contributing to the market growth is the rising prevalence of chronic diseases such as diabetes, rheumatoid arthritis, and multiple sclerosis. As the number of patients requiring long-term medication management continues to increase, the demand for self-administration options like autoinjectors is also on the rise.
Another driving force behind the market expansion is the advancement in technology. Manufacturers are constantly innovating to improve the design and functionality of autoinjectors, enhancing features such as needle safety mechanisms, electronic dose monitoring, and connectivity with mobile applications. These advancements not only improve patient experience but also ensure accurate dosage administration and increase medication adherence.
Moreover, the market is witnessing a shift towards the use of biologic drugs and biosimilars, which require precise and controlled delivery. Autoinjectors are well-suited for these therapies due to their ability to accommodate high-viscosity medications and deliver consistent doses.
In addition to these factors, there is a growing emphasis on patient-centric healthcare, where individuals are empowered to take an active role in managing their own health. Autoinjectors enable patients to self-administer their medications, promoting self-care and improving treatment outcomes.
Overall, the autoinjector market is poised for significant growth as it addresses the evolving needs of patients and healthcare providers. With ongoing technological advancements, expanding applications across various therapeutic areas, and a focus on patient safety and convenience, the market is expected to witness continued expansion in the coming years.
Autoinjector Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Amgen Inc
- Antares Pharma, Inc
- BD
- Amgen
- Teva Pharmaceutical Industries Ltd
- Eli Lilly and Company
- Ypsomed AG
- Novartis AG
- Mylan N.V
- GlaxoSmithKline plc
- Johnson & Johnson Services, Inc
- Bayer AG
Table of Contents
Companies Mentioned
- Amgen Inc.
- Antares Pharma Inc.
- BD
- Amgen
- Teva Pharmaceutical Industries Ltd
- Eli Lilly and Company
- Ypsomed AG
- Novartis AG
- Mylan N.V.
- GlaxoSmithKline plc
- Johnson & Johnson Services Inc.
- Bayer AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | May 2023 |
| Forecast Period | 2023 - 2031 |
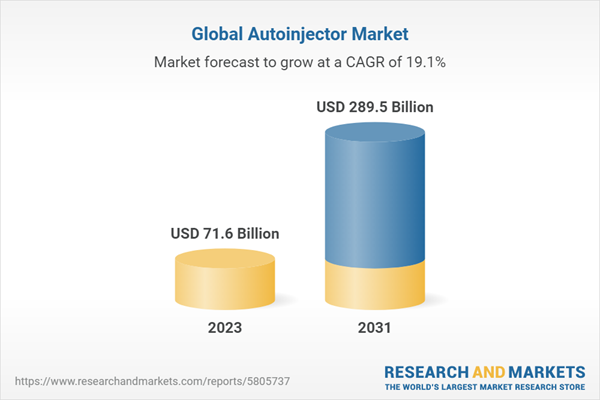
| Estimated Market Value ( USD | $ 71.6 Billion |
| Forecasted Market Value ( USD | $ 289.5 Billion |
| Compound Annual Growth Rate | 19.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |